 UR-144 is a synthetic cannabinoid that’s been sold as a member of the novel psychoactive substances (NPS) market. It produces some cannabis-like effects, though there’s a lack of information about its activity.
UR-144 is a synthetic cannabinoid that’s been sold as a member of the novel psychoactive substances (NPS) market. It produces some cannabis-like effects, though there’s a lack of information about its activity.
We know the drug works at CB1 and CB2 receptors. Those actions seem to result in an effect profile reminiscent of THC and other synthetic cannabinoids. It’s considered more “psychedelic” than some other drugs in the category, but psychedelic-like effects haven’t been reported by all users.
UR-144 = TMCP-018; KM-X1; MN-001, and YX-17
PubChem: 44626619
Molecular formula: C21H29NO
Molecular weight: 311.469 g/mol
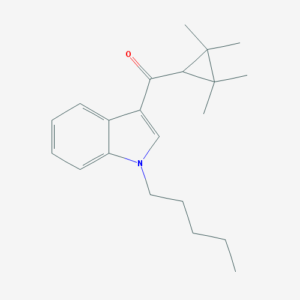
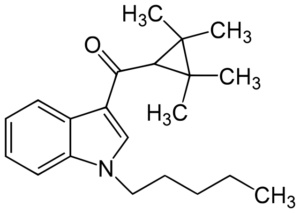
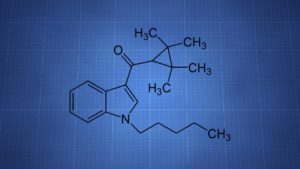
IUPAC: (1-pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone
Contents
Dose
Inhalation (tentative due to a lack of information)
- Range: 2.5 to 20 mg
Most people have been exposed to the drug in the form of “blends” that contain varying levels of UR-144. It’s not wise to use those products.
Timeline
Inhalation (tentative due to a lack of information)
- Total: 1 – 2 hours
- Onset: Under 10 minutes
Experience Reports
Effects
Positive
- Euphoria
- Sedation
- Relaxation
- Mood lift
Negative
- Tachycardia
- Anxiety
- Paranoia
- Unconsciousness
- Memory impairment
Despite being a widely used synthetic cannabinoid, there aren’t many confirmed reports about its effects. It’s therefore only possible to give a brief overview of its apparent effects.
Somewhat dissociative and psychedelic effects (namely visual alterations) have been reported, but it’s not clear what dose provides those effects. The drug is short-lasting in general and the psychedelic effects appear to last under an hour in most cases.
Even though the pharmacological research suggests it is biased towards CB2, it is still clearly psychoactive and has recreational potential.
Animal tests
It was found to cause the following in mice: locomotor suppression, antinociception, hypothermia, and catalepsy.
Chemistry & Pharmacology
Chemistry
It’s classified as a tetramethylcyclopropyl indole. Some of its structure is shared with JWH-018, another synthetic cannabinoid.
UR-144 has a tetramethylcyclopropyl group in place of JWH-018’s naphthyl.
Pharmacology
Tests have repeatedly shown the drug operates at CB1 and CB2 cannabinoid receptors. It’s somewhat unique in the NPS market because it appears to be more potent at CB2, which is generally viewed as a good quality for medical use.
One report provided the following affinity values:
- CB1 – 150 nM
- CB2 – 1.8 nM
The limited pharmacological data suggests it’s a full agonist at both receptors.
A drug discrimination test in mice demonstrated UR-144’s ability to generalize to THC. The discriminative effects were blocked by rimonabant, a CB1 blocker.
CYP3A4 seems to be the most important metabolic route.
Heating, such as in the context of inhaling the drug, leads to a pyrolysis product, 1-(1-pentyl-1H-indol-3-yl)-3-methyl-2-(propan-2-yl)but-3-en-1-one.
History
2006
Abbot Laboratories invented the drug as a possible cannabinoid receptor agonist medication. It was found to be biased towards CB2 over CB1, making it a good potential candidate for medical use.
2010
Data about the drug was published.
2012
Its detection in herbal blends was reported in South Korea. UR-144 appeared to have entered the market sometime between 2011 and early 2012.
The drug’s presence would eventually be noted throughout Europe (e.g. Croatia, Sweden Germany, Finland, Norway, and Hungary) as well as in Japan and the US.
Analysis of samples in Russia found it was the most common synthetic cannabinoid. Similar results were reported in Poland.
Late 2011 – Early 2013
Reported to be among the most common synthetic cannabinoids in Sweden alongside AM-2201, JWH-122, and MAM-2201.
2013
UR-144 and XLR-11 were the most popular synthetic cannabinoids in the US during the first six months of the year. That data was based on information from the National Forensic Laboratory Information System (NFLIS).
The DEA responded to its detection by emergency scheduling it in April 2013.
Legal Status
United States (as of January 2017)
Schedule 1
Controlled (list may not be complete)
Canada, China, Czech Republic, Germany, New Zealand, Russia, and the UK.
Safety
Not enough information exists to know how safe the drug is, particularly with chronic use. It’s wise to use the drug at common doses, infrequently, and without combinations.
Seizures appear to be possible and unconsciousness might occur with high doses.
It should not be taken in the form of “herbal blends.” Using it in that form removes the ability to reliably take common doses.
A couple papers have detailed its impact on driving performance. It’s clear that even seemingly recreational doses can significantly impair driving.
Overdose/severe responses
Case 1
- 26-year-old male
- Presented to the ED with abdominal pain, nausea, vomiting, and lower back pain. His heart rate was 54.
- He admitted to using a product called “Mr. Happy”
- He’d been taking it 2 – 3 times each day for about a year. The last administration was on the morning of his presentation.
- Mr. Happy tested positive for UR-144 and XLR-11
- Tests in the hospital revealed acute kidney injury. The contribution of UR-144 to this isn’t clear.
- Concentration
- Serum at presentation: 0.006 ug/mL
- Serum 3 hours after presentation: 0.003 ug/mL
Case 2
- 22-year-old male
- He was smoking a “joint” of the substance and lost consciousness after taking a few puffs.
- His limbs tightened, followed by convulsions.
- When an ambulance arrived he was unconscious and generalized spasms continued.
- Eventually his Glasgow Coma Score was 10.
- His heart rate was 140 and his speech was incomprehensible.
- Admitted to the hospital
- Reported to be conscious, calm, sleepy, and without any further seizure activity.
- By the next day he was in a good condition and only complained about drowsiness and headache.
- Concentration
- Blood: 0.0061 ug/mL
Fatalities
Case 1
- 36-year-old male
- Took an herbal blend called “Mary Joy Annihilation”
- This was found to contain JWH-122, MAM-2201, AM-2201, and UR-144.
- He collapsed while using it with friends.
- Seizure activity was reported by the time an ambulance arrived.
- He died several hours later despite resuscitation attempts.
Test Videos
References
(2015) Cytochrome P450-mediated metabolism of the synthetic cannabinoids UR-144 and XLR-11
(2014) UR-144 – Critical Review Report
(2014) Quantitative Measurement of XLR11 and UR-144 in Oral Fluid by LC–MS-MS
(2014) XLR-11 and UR-144 in Washington state and state of Alaska driving cases.
(2013) Synthetic cannabinoid use associated with acute kidney injury.
(2013) Toxicological findings of synthetic cannabinoids in recreational users.
(2013) UR-144 (TCMP-018; KM-X1) and XLR11 (5-F-UR-144)
(2013) A fatal case involving several synthetic cannabinoids
(2013) Analysis of UR-144 and its pyrolysis product in blood and their metabolites in urine.
(2012) Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids.