Pregabalin (Lyrica) is a medication in the gabapentinoid class. It has anticonvulsant, anxiolytic, and analgesic properties. As such, it’s used for epilepsy, neuropathic pain, anxiety, and other conditions.
Despite being a GABA derivative, it doesn’t appear to be operating through a GABA-related mechanism.
The substance is also used recreationally due to its euphoric, anxiolytic, and pro-social effects.
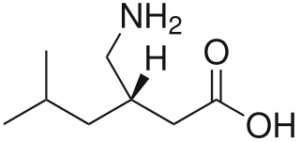
Pregabalin = Lyrica; (S)-3-isobutyl-GABA
PubChem: 5486971
Molecular formula: C8H17NO2
Molecular weight: 159.229 g/mol
IUPAC: (3S)-3-(aminomethyl)-5-methylhexanoic acid
Contents
Dose
Medical
Range: 150 – 600 mg/d
Typically it’s started at ~150 mg/d (split into multiple doses) and that dose increases weekly until an ideal response is achieved.
Non-medical
Light: 150 – 300 mg
Common: 300 – 600 mg
Strong: 600 – 900 mg
While some people report using over 1 gram, it’s not a great idea. We know less about its safety at that dose and the severity of negatives is known to increase.
Timeline
Oral
Total: 6 – 10 hours (though some effects can persist for another 5 hours)
Onset: 30 – 60 minutes
Even though the onset should be under an hour, a lot of people in recreational settings report a delay. The effects could take 1.5 – 2.5 hours to really become apparent.
Experience Reports
Effects
Medical
It’s primarily used for neuropathic pain, fibromyalgia, seizures, and anxiety. Though it does have other applications.
Some of the reasons for its widespread use are its fast onset of efficacy, low drug-drug interaction potential, and its relatively high safety level.
Along with helping people’s core condition (e.g. neuropathic pain), it can improve secondary insomnia and anxiety.
There have been criticisms of its widespread use, however. Part of the critique comes from those who are worried about its abuse potential.
Seizures
Pregabalin is mainly used as an adjunct therapy for partial seizures, meaning it’s given to people who aren’t receiving adequate benefit from their existing medications.
Studies have found it can reduce the frequency of partial seizures, particularly at 300 – 600 mg/d. According to (Kalviainen, 2008), it may aggravate myoclonus and myoclonic seizures, so it should be avoided in those populations.
Compared to other adjunct treatments, it might be superior to gabapentin and is similar in efficacy to levetiracetam.
As a monotherapy, it may be inferior to lamotrigine and topiramate.
Reviews
(Zhou, 2012) – Reviewing its use as a monotherapy
- Only two short-term studies available with 753 total participants, one comparing it to lamotrigine and one to gabapentin.
- Both dealt with partial seizures: one for newly diagnosed partial seizures (vs lamotrigine) and one for refractory partial epilepsy in hospitalized patients (vs gabapentin.)
- Pregabalin was found to be inferior to lamotrigine in terms of efficacy, as measured by time to withdrawal due to inadequate seizure control following dose stabilization.
- No significant differences in terms of safety-related outcomes; similar tolerability across medications.
- Pregabalin appeared to be superior to gabapentin.
- The clinical disadvantage of pregabalin vs. lamotrigine was higher in Asia than in North America, so ethnic differences should be investigated.
(Pulman, 2014) – Cochrane review of its efficacy as an adjunct partial epilepsy treatment
- Six industry-sponsored studies with 2009 participants were available.
- Dose: Ranging from 50 mg/d to 600 mg/d
- Primary outcomes
- 50% or greater seizure reduction was significantly more likely with pregabalin vs. placebo.
- And there appeared to be a dose-response, with the odds of response doubling from 300 mg/d to 600 mg/d
- Significantly associated with seizure freedom.
- Adverse: Ataxia, dizziness, drowsiness, and weight gain.
- Evidence had a low/unclear risk of bias due to potential publication bias.
- Quality of evidence was moderate.
Studies
(Zaccara, 2014) – Comparing pregabalin to levetiracetam as an adjunct treatment
- Across 71 centers in Western and Eastern Europe, South and Central America, and Asia.
- Design: Randomized, double-blind, flexible-dose, two-arm, parallel-group, noninferiority
- 6-week baseline screening phase
- 4-week dose-escalation phase
- 12-week maintenance phase
- Patients entered the trial with stable doses of 1 or 2 other medications
- They had a minimum of 4 partial seizures during the 6-week baseline phase and no 28-day period without seizures.
- Results
- 509 randomized: pregabalin (254) and levetiracetam (255)
- Of these, completion of maintenance phase was 81.9% for pregabalin and 82.4% for levetiracetam
- Most common other medications: carbazepine, valproic acid, topiramate, oxcarbazepine
- Median dose: 450 mg/d for pregabalin and 2000 mg/d for levetiracetam
- Primary endpoint of 50%+ reduction in 28-day seizure rate from baseline to end of maintenance phase
- 59.1% for pregabalin and 58.8% for levetiracetam
- Pregabalin wasn’t inferior to levetiracetam
- Secondary endpoint of seizure-free for the final 28 days of the maintenance phase
- Lower in pregabalin group (19.9%) than levetiracetam (27.6%); non-significant difference
- Post hoc analysis of seizure-free for duration of maintenance phase
- Lower in pregabalin group (8.4%) than levetiracetam (16.2%); significant difference
- 509 randomized: pregabalin (254) and levetiracetam (255)
- Safety
- Most common adverse effect was drowsiness: 31.1% with pregabalin vs. 28.6% with levetiracetam
- Dizziness more common in pregabalin: 22% vs. 15.3%
- Weight gain more common in pregabalin: 9.4% vs. 2.0%
- Nausea more common in levetiracetam: 1.2% for pregabalin and 5.9% for levetiracetam
- Discontinuations were more common in the dose escalation period with pregabalin and more common during maintenance with levetiracetam.
- Could be from early drowsiness/dizziness occurring with pregabalin, while slower onset mood changes could be a problem with levetiracetam.
- COI: YES; sponsored by Pfizer
Animal research
- Maximal electroshock test in rats: ED50 of 1.8 mg/kg (oral)
- Estimated Cmax of 1.6 ug/mL, which is within the therapeutic range seen in humans.
- It’s been shown to be effective against partial seizures & generalized tonic-clonic seizures.
- But not against absence seizures.
- Maximal electroshock studies in mice: Prevented tonic extensor seizures
- Prevents generalized tonic-clonic seizures induced by sound in DBA/2 mice.
- Prevents threshold clonic seizures induced by chemical convulsants like pentylenetetrazol.
Neuropathic pain
Pregabalin is effective for certain kinds of neuropathic pain, including postherpetic neuralgia, diabetic neuropathy, and central neuropathic pain. A lack of impact was found on HIV-associated neuropathy.
The most reliable benefits are seen with 200 – 600 mg/d.
Compared to gabapentin, there’s at least a trend towards superior responses with pregabalin.
Reviews
(D’Arcy, 2017)
- Meta-analysis of 25 pregabalin studies; found 18 were positive based on their primary endpoint.
- The overall NNT for all trials was 7.7.
- The meta-analysis gave the drug a GRADE rating of “strong” and proposed it as a first-line therapy for neuropathic pain.
- Pain relief tends to be dose-dependent, with 600 mg/d offering more than 300 mg/d.
- Literature supports pregabalin improving sleep secondary to the primary endpoint, but it’s not approved for insomnia specifically.
- Onset
- Typically there are benefits within 1 week, but those who achieve a 30 or 50% reduction in pain do so within 3-4 weeks, though that could be partly due to the starting weekly dose being just 150 mg/d.
- COI: Sponsored by Pfizer
(Wiffen, 2016) – Cochrane review of its use in neuropathic pain and fibromyalgia
- Currently, evidence exists in support of pregabalin being helpful in diabetic neuropathy, postherpetic neuralgia, central neuropathic pain, and fibromyalgia.
- Only a minority of people achieved acceptably good pain relief with pregabalin, but it can be helpful.
Fibromyalgia
It appears effective at improving pain and sleep in fibromyalgia, with a potentially less reliable or significant impact on fatigue. (Crofford, 2005) report it could improve sleep and pain.
Reviews
(Lyseng-Williamson, 2008)
- Efficacy
- Generally associated with significant pain improvements, particularly at 450 and 600 mg/day, but it can also be effective at 300 mg/day.
- Some trials have found it’s significantly better at a higher, but not lower dose. And some results have shown 450 mg/d is effective, but not 300 mg/d or 600 mg/d.
- When improvement is seen, it’s typically sustained for months and even longer.
- Changes in pain scores are correlated with improvements in sleep quality and, to a lesser extent, with improved fatigue.
- There’s little correlation with mood, anxiety, and/or depressive symptoms.
- Secondary endpoints
- Associated with improvements in sleep at 300, 450, and 600 mg.
- Though it also comes with significantly greater drowsiness.
- Associated with improvements in sleep at 300, 450, and 600 mg.
- Tolerability
- Over 5% of patients experience (with a rate at least 2-fold that in placebo): dizziness, drowsiness, weight gain, blurred vision, dry mouth, constipation, peripheral edema, euphoria, increased appetite, attention disruption, and balance disorder.
- Based on three multicenter trials of 8, 13, and 14 weeks in US, the top rates were:
- Dizziness: 38% vs. 9%
- Drowsiness: 20% vs. 4%
- Weight gain: 11% vs. 2%
- Blurred vision: 8% vs 1%
- Adverse events led to discontinuation of treatment in 19% of pregabalin patients and 10% of placebo across multiple US short-term trials.
- Adverse events are more common with higher doses and are sometimes only transient.
- Dizziness and drowsiness lead to ~6% and 3% discontinuation, respectively, compared with under 1% in placebo.
- Weight gain
- 11% vs 2% for having a greater than 7% increase in weight.
- Sometimes associated with elevated creatine kinase levels, decreased platelet count, and prolonged PR intervals.
- Has been connected to angioedema and hypersensitivity, which should lead to discontinuation.
- Based on three multicenter trials of 8, 13, and 14 weeks in US, the top rates were:
- Over 5% of patients experience (with a rate at least 2-fold that in placebo): dizziness, drowsiness, weight gain, blurred vision, dry mouth, constipation, peripheral edema, euphoria, increased appetite, attention disruption, and balance disorder.
- COI: None
Generalized anxiety disorder
Research has found it’s more effective than placebo, but it’s not yet clear if it should be a first-line treatment. When it works, it has a relatively fast onset of efficacy and a similar level of impact on psychic and somatic symptoms.
Some studies have found benzodiazepines aren’t really superior to a higher dose of pregabalin and that it also comes close to SSRIs/SNRIs. While other research has found a pretty small clinical effect on certain scales.
It’s approved in Europe, but not the US, for this condition.
There are some patients who benefit from pregabalin while being resistent to other treatments.
Reviews
(Wensel, 2012)
- 8 double-blind, placebo-controlled studies
- 6/8 were short-term (4 – 8 weeks)
- 5/8 also had active control: lorazepam, alprazolam, or venlafaxine.
- Dose: Ranged from 150 mg/d to 600 mg/d
- Results
- Pregabalin patients experienced significantly higher HAM-A declines
- Mean range of 9.24 – 14.7 for pregabalin vs. 6.82 to 11.7 with placebo.
- Significant benefits could arrive within a week.
- Response was generally comparable to the active comparators.
- 3/8 trials showed non-significant benefits for somatic symptoms, while psychic subscale was non-significant in just 1 trial (with 150 mg/d)
- Clinical response (50% decline from baseline) was achieved less often
- 47% – 61% rate with pregabalin vs. 34% – 44% with placebo.
- At times, this was significant, other times it was non-significant.
- No clear dose-dependency
- E.g. One non-significant trial with 600 mg/d yielded a 59% response with pregabalin vs. 44% with placebo
- But another significant trial with 600 mg/d yielded 53% with pregabalin vs. 34% with placebo.
- Pregabalin patients experienced significantly higher HAM-A declines
- Adverse
- Mainly drowsiness, dizziness, and dry mouth. — Could be better tolerated by some than SSRIs/SNRIs.
- Long-term efficacy evaluated in 1 study
- Up to 6 months evaluated
- 624 initial participants receiving 450 mg/d for 8 weeks
- Patients entered double blind 24-week phase if they had a clinical HAM-A response at or below 11
- 168 patients than received 450 mg/d still, while 170 received placebo
- Pregabalin significantly delayed the onset of relapse vs. placebo
- At 6 months:
- 42% pregabalin relapsed vs. 65% with placebo.
- Pregabalin patients had a HAM-A of 7.2 vs. 11.8 with placebo.
- Small, but significant difference also seen with CGI-Severity scale.
- COI: None
(Baldwin, 2013)
- 6 fixed dose studies of 4-6 weeks; 2 flexible dose 8-week studies incl. one in elderly patients; single 6-month fixed dose relapse prevention study
- Based on the available data, there is evidence supporting pregabalin’s ability to acutely treat GAD and prevent relapse.
- Though an analysis of controlled trials found an overall mean effect size of 0.39, which is small.
- Some data also supported its use vs. placebo in reducing anxiety in patients prior to undergoing dental or orthopedic procedures, generally with an onset of effect within a few hours.
- Post-hoc analysis indicates that response to pregabalin within 2 weeks is associated with a 5.3-fold odds ratio of responding to treatment; while just one quarter of patients with no onset by the second week will have a response by study endpoint.
- Generally no evidence for dose-response so far, with the exception being that 150 mg may not be as effective as 200 to 600 mg.
- Associated with significant reduction in sleep disturbance with 300 to 600 mg/d.
- It may also reduce depressive symptoms in those with comorbid depression.
- Most adverse effects have an onset of under 1 week; they decline generally within 3 weeks
- COI: None
(Montgomery, 2006)
- Efficacy
- Onset
- The greatest reason for continued benzodiazepine use for GAD appears to be its favorable onset period (~1 week) vs SSRIs/SNRIs.
- With pregabalin, the onset is also around 1 week.
- Pregabalin (300, 450, and 600 mg) offers similar improvements by Week 1 to alprazolam.
- Offers greater improvement than venlafaxine at Week 1
- The greatest reason for continued benzodiazepine use for GAD appears to be its favorable onset period (~1 week) vs SSRIs/SNRIs.
- Psychic and somatic improvements
- Offers significant and basically equivalent efficacy for psychic and somatic symptoms.
- Significant improvement in HAM-A anxious mood/worry and HAM-A tension, with the majority of patients showing 50%+ reduction in severity of both symptoms at 4-6 weeks.
- Subgroups
- Elderly
- Appears just as effective up to 8 weeks.
- Illness severity
- 300-600 mg appears effective in both moderate and severe groups without a dose-response effect.
- In fact, 150 mg was effective in severe patients but not moderate.
- Concomitant subsyndromic depression
- HAM-D scores improved significantly at 150-600 mg, though 300+ mg is most reliably effective at reducing moderate depressive symptoms.
- Elderly
- Insomnia
- Improves sleep scores.
- VS other medications
- Reduces psychic GAD symptoms similarly to SSRIs/SNRIs and greater than benzodiazepines.
- Reduces somatic GAD symptoms greater than SSRIs/SNRIs.
- Onset
- Safety
- Lab/ECG/other
- Doesn’t appear to be associated with clinically significant HR, BP, respiration, or ECG changes.
- Just a nonsignificant PR interval increase of 3-6 ms at 300 to 600 mg/d.
- Peripheral edema seen in around 1.9%, but only severe in around 0.1% and not associated with impaired hepatic or renal function, or weight, BP, HR, or ECG changes.
- Withdrawal
- In rats, discontinuing supratherapeutic doses only led to mild behavioral withdrawal signs.
- In humans, a long-term GAD trial of 32 weeks showed that rapid discontinuation led to minimal-to-no withdrawal signs (PWC only increased 3 points.)
- Whereas withdrawal symptoms are clear with benzodiazepines with rapid discontinuation (PWC increase of 24 points)
- Lab/ECG/other
- COI: YES; consultant for companies
Postoperative pain
Variable effects of pregabalin have been seen on postoperative pain. It might be able to reduce opioid use and pain, but more research is needed. Even when it does offer some level of change, it might not be clinically significant.
Given it can raise the chance of some side effects (e.g. sedation), it may or may not be useful. Certain populations, like the elderly, may be better off avoiding it.
Some reports suggest it can reduce the risk of nausea and vomiting.
Positive reports exist for its administration prior to tonsillectomy, nasal surgery, and lumbar spinal surgery.
Reviews
(Zhang, 2011)
- 11 randomized, controlled clinical trials with 899 patients (521 receiving pregabalin)
- Data didn’t reliably shown a significant decline in pain scores, though it did seem to reduce opioid use.
- No significant difference in pain scores at 2 hours or 24 hours.
- Some studies showed a significant analgesic effect, but not an overall strong one.
- It reduces postoperative vomiting, though it came with a higher rate of visual disturbance.
- COI: None
Studies
(Bekawi, 2014) – Positive results
- 90 people across three groups undergoing laparoscopic cholecystectomy
- Pregabalin
- 150 mg – 2 hours before surgery & 12 hours after operations & twice daily for 2 days.
- Gabapentin
- 1200 mg – 2 hours before surgery & 12 hours after operation & 400 mg three times daily for 2 days
- Control
- Pregabalin
- Results
- VAS pain scores
- Not significantly different between groups. All had a significant decline in VAS scores over time.
- The pregabalin group had the lowest mean scores vs. gabapentin or control groups.
- 24-hour pethidine consumption
- Significant difference in the percentage requiring pethidine
- 100% in control
- 36.7% in gabapentin
- 26.7% in pregabalin
- Significant difference in the percentage requiring pethidine
- Side effects
- Sedation
- Pregabalin group had a lower sedation score at different time intervals compared to gabapentin or control groups.
- Neither pregabalin nor gabapentin ever exceeded a mean score of 2, while control was in the 3-4 range.
- Nausea and vomiting
- Control group had significantly more patients with nausea and vomiting.
- 86.7% in control
- 43.3% in gabapentin
- 30% in pregabalin
- No significant differences in moderate to severe nausea/vomiting between groups.
- Control group had significantly more patients with nausea and vomiting.
- Postoperative dizziness
- Control group had significantly more patients with moderate to severe dizziness
- 53.3% in control
- 0% in pregabalin and in gabapentin
- No significant difference for mild/no dizziness.
- Control group had significantly more patients with moderate to severe dizziness
- Sedation
- Satisfaction
- Overall patient satisfaction with pain management was significantly higher in the pregabalin vs. gabapentin or control groups.
- VAS pain scores
- COI: None
Chronic postoperative pain
There’s even less evidence in favor of it being able to reduce chronic postoperative pain, yet there’s been some interest in using it for that reason. Some unpublished data from Pfizer was negative for that indication.
One investigation found pregabalin wasn’t effective, while ketamine was.
Pain
No real evidence exists that supports pregabalin as a go-to medication for acute pain. Other pain-related uses may exist.
Reviews
(Gurusamy, 2016) – Cochrane review of its use in pancreatic pain from chronic pancreatitis
- Only one study, funded by Pfizer, was available.
- 64 total participants: 34 with pregabalin and 30 with placebo
- Low risk of bias; quality of evidence was low to moderate
- Found pregabalin offered a reduction in pain scores and opioid use vs. placebo.
- Though there were also more adverse events.
Social anxiety
Like with GAD, 150 mg doesn’t appear very effective, but 300 – 600 mg/d might be effective.
Studies
(Pande, 2004)
- Double-blind, placebo-controlled at 6 US centers
- 47 with pregabalin 600 mg/d, 42 with pregabalin 150 mg/d, and 46 with placebo
- Results
- 600 mg/d led to Significantly greater baseline to endpoint LSAS total reduction vs. placebo
- 150 mg/d wasn’t significant
- Response began at 1 week for LSAS and lasted throughout study.
- At 600 mg/d, patients reported significantly less fear, avoidance, social fear, and social avoidance.
- Higher rate of response with pregabalin.
- Also HAM-A benefits, but the change wasn’t significant.
- Most common negatives: headache, dizziness, and drowsiness.
- 8 had one or more sexual adverse effects
- With pregabalin: decreased libido, anorgasmia, impotence, and delayed ejaculation.
- Weight gain
- 600 mg/kg: Mean of 1.7 kg
- 150 mg/kg: Mean of 0.6 kg
- Placebo: Mean of 0.1 kg loss
- 600 mg/d led to Significantly greater baseline to endpoint LSAS total reduction vs. placebo
- COI: Yes; supported by Parke-Davis
Insomnia
It often has a positive impact on sleep across conditions.
Both healthy and treatment populations have reported improved sleep from the substance.
It can increase slow-wave sleep, improve sleep maintenance, reduce Stage 1 sleep, and potentially improve sleep onset. Though it does reduce REM.
Reviews
(Roth, 2013)
- Investigated the impact of pregabalin on sleep in partial epilepsy, neuropathic pain, restless leg syndrome, and fibromyalgia.
- Results
- It consistently improved patient-reported sleep quality in a dose-related manner, with the main exception being with mixed reports for partial seizures.
- Benefits were usually sustained.
- Could improve wake time after sleep onset, the number of awakenings, and time spent in slow-wave sleep.
- It also decreased Stage 1 sleep.
- Decreased latency to sleep onset can be seen, but the greatest impact of pregabalin is on sleep maintenance.
- It therefore improves maintenance and the “depth” of sleep.
- Supported by patient reports and PSG readings.
- Benefit could come from direct pro-sleep effect and from reducing the symptoms of the primary condition.
- COI: Yes. Funding from companies & employees of Pfizer.
PTSD
Very little research exists other than a little bit of evidence in favor of its use as an adjunct therapy.
Studies
(Pae, 2009) – Open-label trial for accident-related PTSD
- 9 participants with chronic PTSD
- Other therapy was continued without dose change.
- Dosing
- Began at 75 mg/d and flexibly titrated upwards or downward
- Maximal target dose of 450 mg/d
- Results
- Results
- Did have some encouraging results.
- Week 2
- No statistical improvement on SPRINT, PGI-S, VAS-pain, MADRS, or SDS.
- Week 4
- Statistically significant reductions in SPRINT, PGI-S, VAS-pain, MADRS, and SDS.
- Week 6
- Statistically significant reductions in SPRINT, PGI-S, VAS-pain, MADRS, and SDS
- 5/9 patients had a greater than 50% decline on PGI-S
- 6/9 patients had a greater than 50% decline on VAS-pain
- Though not that level of effect for other measures.
- Tolerability
- Well-tolerated, negatives declined within 2 weeks, and no severe adverse effects.
- Results
- COI: Yes.
Other anxiety
Studies
(Nutt, 2009) – Showing an impact from a single dose on dental procedure anxiety
- 89 participants: 27 in pregabalin (150 mg), 31 in alprazolam (0.5 mg), and 31 in placebo.
- Subjects in pregabalin group had a more chronic history of dental anxiety, though overall severity of dental anxiety (by history) was similar between groups.
- Baseline VAS-Anxiety scores were higher in the pregabalin group compared with alprazolam.
- Results
- VAS-anxiety
- Pregabalin led to a reduction in anxiety, though not significant vs. placebo.
- Whereas alprazolam led to significant VAS-anxiety decline from 2.5 hours postdose to 4 hours postdose.
- TOAS
- Pregabalin led to greater improvement in anxiety vs. placebo, showing trend towards significance at 2.5 h, and then achieving significance from 3 h to 4 h.
- Alprazolam led to significantly higher TOAS scores vs. placebo at all assessment points from 2 h to 4 h.
- TOAS (proportion of responders)
- At 2 hours, the proportion showing response was significantly higher with alprazolam vs. placebo, but not significantly higher with pregabalin.
- Pregabalin led to significantly higher VAS-Sedation scores from 2.5 h to 4h vs placebo.
- VAS-anxiety
- Interpretation
- Results show some anxiolytic action from a single dose, though it’s less significant than with alprazolam.
- And alprazolam may offer a faster onset.
- Results show some anxiolytic action from a single dose, though it’s less significant than with alprazolam.
- COI: Yes; funding by Pfizer.
Drug dependence and/or addiction
Research and anecdotal reports suggest pregabalin can reduce the withdrawal symptoms and craving associated with certain drug dependencies. It’s specifically been evaluated for opioid, alcohol, and benzodiazepine dependence.
In informal settings, a lot of opioid users find it’s helpful at alleviating withdrawal symptoms.
Reviews
(Oulis, 2012) – Use for alcohol and benzodiazepine dependence
- Overall, we need more research. But current findings suggest 150-600 mg/d might be a safe option for treating dependence to alcohol and benzodiazepines.
- Alcohol
- Some preclinical animal studies have found it can reduce operant conditioning with alcohol and reduce alcohol drinking.
- It also reduced behavioral and seizure symptoms during alcohol withdrawal in animals.
- Human studies
- Four pieces of research from an Italian group found preliminary evidence that it may help in the treatment of alcohol dependence by reducing craving and reducing withdrawal symptoms.
- However, other studies, including a double-blind placebo-controlled one in 41 in-patients found it didn’t outperform placebo in terms of anti-withdrawal effects.
- Benzodiazepine
- Similar situation to alcohol; there have been results showing statistically significant benefits of pregabalin in reducing somatic and psychic anxiety, severity of withdrawal symptoms, and patient and clinician-rated assessments of global improvement.
- But more research is needed.
- COI: None
(Freynhagen, 2016) – Reviewing role in multiple drug dependencies
- Opioid withdrawal symptoms
- Case report evidence
- (Scanlon, 2014) reported successful detox in opioid-dependent individual after 6 days of pregabalin 300 mg.
- At the end of assessment, there were no withdrawal symptoms.
- Symptoms like anxiety, insomnia, and abdominal cramping were better controlled vs. their previous withdrawals.
- (Kammerer, 2012) reported successful use of pregabalin in someone who failed replacement therapy with buprenorphine for heroin dependence.
- Heroin intake and withdrawal symptoms were ameliorated with pregabalin for 2-3 days at 300 mg/d.
- (Kontoangelos, 2013) reported on a patient using codeine and fentanyl for pain.
- Pregabalin began at 75 mg/d and increased over 2 weeks to 300 mg/d while withdrawing from codeine and fentanyl.
- Pain symptoms progressively improved during this time and there were no opioid withdrawal symptoms
- (Scanlon, 2014) reported successful detox in opioid-dependent individual after 6 days of pregabalin 300 mg.
- Preclinical
- (Hasanein, 2014) Rats made opioid dependent show dose-dependent reduction of naloxone-induced withdrawal symptoms in the presence of pregabalin.
- Case report evidence
- Benzodiazepines and zolpidem
- Uncontrolled, observational
- (Bobes, 2012) pregabalin at a mean of 315 mg/d by Week 12 was found to significantly reduce benzodiazepine withdrawal symptoms.
- Anxiety symptoms improved 69% and tolerability rated at 80-90%.
- (Bobes, 2012) pregabalin at a mean of 315 mg/d by Week 12 was found to significantly reduce benzodiazepine withdrawal symptoms.
- Pharmacoepidemiological study
- (Bramness, 2010) Pregabalin use monitored in patients with psychiatric disorders, epilepsy, neuropathic pain, or a non-specified condition.
- 14.7 – 27.9% stopped using benzodiazepines after starting pregabalin.
- 48% of those with psychiatric conditions reduced benzodiazepine consumption
- (Bramness, 2010) Pregabalin use monitored in patients with psychiatric disorders, epilepsy, neuropathic pain, or a non-specified condition.
- Zolpidem prospective open-label trial
- (Cho, 2014) 40 patients with mean hypnotic use duration of 2.6 years; 75% using zolpidem
- 52.5% successfully withdrew from hypnotic medication after 8 weeks of pregabalin 75-300 mg/d
- Significant improvement in withdrawal symptoms, sleep quality, and insomnia severity.
- (Cho, 2014) 40 patients with mean hypnotic use duration of 2.6 years; 75% using zolpidem
- Zolpidem case report
- (Oulis, 2011) Individual heavily used zolpidem with up to 1500 mg/d
- Able to discontinue with pregabalin 600-900 mg/d on two occasions, with no noticeable discontinuation or craving symptoms.
- (Oulis, 2011) Individual heavily used zolpidem with up to 1500 mg/d
- Uncontrolled, observational
- Alcohol
- Mixed results
- Preclinical
- (Becker, 2006) – In mice chronically given ethanol, pregabalin dose-dependently reduced behavioral convulsions vs. placebo.
- (Martinotti, 2008) (open-label)
- Pregabalin led to significant improvement in withdrawal symptoms and alcohol craving.
- (Di Nicola, 2010) (open-label)
- Pregabalin at 200-450 mg/d led to significant reduction in withdrawal symptoms and craving scores.
- (Martinotti, 2010) – Randomized, single-blind study comparing pregabalin with tiapride and lorazepam
- After 14 days of treatment, all groups had significant improvement in withdrawal symptoms, craving, quality of life, and comorbid psychiatric symptoms.
- Significantly more people remained alcohol-free in pregabalin vs. tiapride or lorazepam group
- Significantly more people remained on treatment in pregabalin vs. tiapride group, but not lorazepam group.
- (Forg, 2012) – Randomized, double-blind, placebo-controlled
- Both pregabalin and placebo significantly reduced craving and withdrawal symptoms
- No significant difference in favor of pregabalin
- Cannabinoid
- Preclinical
- (Aracil-Fernandez, 2013) – Mice tolerant to cannabinoids following CP-55,490
- Pregabalin improved withdrawal symptoms, including anxiety-like symptoms and reduced motor activity, 1 to 3 days after cessation of cannabinoid.
- (Aracil-Fernandez, 2013) – Mice tolerant to cannabinoids following CP-55,490
- Preclinical
- COI: Yes; speaking fees and consultancy fees
Other
It could have some efficacy in OCD, bipolar disorder, depression, and schizophrenia.
Restless legs syndrome
It was found to offer a similar reduction to a dopamine agonist in the International Restless Leg Syndrome score. But it had a lower improvement on the Periodic Limb Movement Index.
Negatives in medical settings
Drowsiness and dizziness are the most common negatives. Both tend to decline over time and are dose-dependent. Other CNS depressants can exacerbate those negatives.
Of all the reasons for discontinuation, drowsiness is one of the most common.
Pregabalin has a similar adverse effect profile to gabapentin, with these being the most common negatives from both: dizziness, drowsiness, ataxia, peripheral edema, dry mouth, fatigue, and blurred vision.
Weight gain
A minority of patients will experience a problematic level of weight gain. One review found about 1-in-6 people had a greater than 7% increase in weight after 2 to 12 months of treatment.
VS other drugs
Compared to lamotrigine, it’s associated with a higher rate of drowsiness and weight gain.
Compared to gabapentin, there’s no difference for dizziness, drowsiness, fatigue, nausea, or ataxia.
Cognitive and psychomotor
It doesn’t typically impair daytime functioning, but self-reported concentration and attention difficulties sometimes show up
With up to 450 mg/d, there’s no clear detrimental effect on reaction time, vigilance, or serial memory scanning. Though it may have a small, transient impact on CNS arousal, divided attention, and sedation.
There’s less impact overall than what’s seen with benzodiazepines.
Reviews
(Zaccara, 2011)
- 38 studies examined – only double-blind, RCTs
- 11,918 subjects total – 8,235 receiving pregabalin
- Across neuropathic pain, fibromyalgia, epilepsy, GAD, social anxiety disorder, and panic disorder.
- Some had flexible dosing, others were fixed.
- Duration of 4-14 weeks.
- Results
- Some of the adverse effects strongly associated with pregabalin were balance disorder, euphoria, incoordination, ataxia, and edema.
- Very rare severe responses included edema w/ reduced platelet count and hypervolemia, and a separate one with subacute myocardial infarction.
- Dose-response
- Presenting by 150 mg/d: Dizziness, ataxia, drowsiness, edema, dry mouth
- 300 mg/d: Vertigo, incoordination, blurred vision, ambylopia, confusional state, disturbed attention, thinking abnormal, euphoria, asthenia, peripheral edema, constipation
- 450 mg/d: Balance disorder and fatigue
- 600 mg/d: Diplopia and tremor
- Overall, 20 AEs were significantly associated with pregabalin treatment.
- 16/20 were cognition/coordination issues
- COI: Yes; speaker or consultant fees.
Non-medical
Positives
- Euphoria
- Mood lift
- Sedation
- Analgesia
- Muscle relaxation
- Anxiolysis
- Light to moderate entactogen effects (pro-social, greater empathy, greater appreciation)
- Light to moderate dissociation
- Physical euphoria (Pleasant sensations. Feelings of warmth and/or energy. Altered tactile perception.)
- Increased self-confidence
- Calmness
Negatives
- Dizziness
- Drowsiness
- Amnesia – [Typically just minor and not prominent at common doses. Actual anterograde amnesia, like is possible with ethanol and benzodiazepines, isn’t a notable effect.]
- Impaired motor control
- Cognitive impairment
- Nausea
- Muscle twitches – [Unclear occurrence. Not really reported in medical settings.]
Even patients using medical doses sometimes report recreational effects like euphoria, slight dissociation, anxiolysis, and mental stimulation. But the full recreational effects are dose-dependent, with common to strong amounts providing the most reliable activity.
It’s clearly a CNS depressant, but that’s not the full story. Many users report a mental stimulation effect, including increased motivation and a desire to work on things. That effect isn’t accompanied by normal stimulant-like physical stimulation. Some people get more done when on the drug.
Unless a very large dose is used, it doesn’t tend to cause overwhelming sedation.
Due to its fairly significant recreational potential, people compare it more to ethanol and GHB than to benzodiazepines.
The dissociative effects aren’t as significant as with real dissociatives. Higher doses are more likely to result in that kind of activity. It can contribute to daydreaming and anxiolysis.
Switching from medical to non-medical use is most common in those with a history of drug addiction.
People also like the drug because it tends to lack negative after effects, instead offering a positive afterglow, albeit with some impaired motor control and sedation.
Polydrug
It can potentiate the positives and negatives of opioids, ethanol, and benzodiazepines.
Some people have combined it with psychedelics and MDMA due to the potential for lower anxiety and mood improvement. This is similar to how some people use phenibut.
Studies
(Schifano, 2011) – Examining online recreational reports to figure out effects.
- Data from Jan 2008 to Aug 2010
- It was often described positively with effects like:
- Similar to alcohol/GHB/benzodiazepine and some euphoria.
- Slight entactogen properties (perhaps more on GHB than benzo side).
- DXM-like dissociation
- Reduced withdrawal symptoms from opioids and reduced cravings
- Some described it like an opioid with DXM-like dissociation, and generally said it was better than gabapentin.
- Often people reported using much more than recommended: At least over 600 mg and even over 5 grams.
- Dose-dependent effects
- 600 mg – Disorientation, slurred speech, double/blurred vision, disinhibition, talkativeness, anxiolysis, and perhaps visual alterations/hallucinations.
- 900 mg – Strong drunkenness feel, difficulty walking, altered color perception, some minor euphoria.
- 1200 mg – Drowsiness, more euphoria, some entactogenic effects.
- 1500 to 5000 mg – Very heavy uncontrollable drowsiness, hallucinations, greater euphoria, quite dissociative, disinhibition, and some potential FOR anxiety.
- Some people dislike anything over 900 mg at once due to strong sedation.
(Schjerning, 2016) – Examining the potential abuse of pregabalin among those with prescriptions in the Netherlands.
- Looking at all Danish users of pregabalin from 2004 to 2013.
- 80,868 total patients
- Of those, 4090 (9.6%) received over 600 mg/d for 6 months, and 2765 (6.5%) for over 12 months.
- In total, just 276 (0.65%) got over 1200 mg/d for 6 months, and 137 (0.33%) for over 12 months.
- Prescriptions for antipsychotic drugs and benzodiazepines were associated with increased risk of using pregabalin at above-licensed doses.
- This data led to a Lorenz-1 value of 6.1%, which doesn’t indicate very high abuse potential (e.g. little/no abuse drugs like statins are under 5%, while benzos/opioids are typically over 10%)
- At least at a population level (not specifically among those with a history of opioid use), the trend towards abuse doesn’t seem massive.
- COI: YES; research grants and speaking fees.
Case reports
(Carrus, 2012) – Two cases of people using it recreationally after receiving a prescription.
- Case 1
- 32-year-old
- History of antisocial personality disorder and benzodiazepine, cocaine, and ecstasy abuse.
- For neuropathic pain, he received pregabalin
- Initial: 150 mg/d
- By day 12: 600 mg/d
- Over the following 4 weeks, he increased the dose to an alleged 4500 mg/d
- Also using alcohol and cannabis
- When he tried to discontinue pregabalin on multiple occassions
- Anxious, irritable, aggressive
- Positive effects reported with pregabalin: more relaxed, more empathetic, and with “very good drive.”
- Case 2
- 33-year-old
- History of “abusing” alcohol and ecstasy.
- Referred for bipolar and GAD
- Given olanzapine 10 mg/d and started at 75 mg/d pregabalin
- Pregabalin increased to 300 mg/d by Day 8
- During the first few days of pregabalin intake
- Reported feeling increasingly relaxed, emphasizing the effects were “very similar to those of cannabis.”
- He then gradually increased up to 1500 mg/d over a 4-week period
- Felt like he was “under the influence of an anesthetic” and “very light.”
- Over time, he eventually developed severe muscle pain.
- CPK values unavailable, but tentative diagnosis of myositis was made.
- Naranjo Probability Scale found pregabalin was the probable cause of the myositis.
- When he stopped pregabalin, the muscle pain disappeared over a week.
Other effect studies
(Zacny, 2012) – Interaction with oxycodone
- Double-blind, randomized, placebo-controlled, crossover
- 16 participants
- Conditions
- Placebo
- 75 and 150 mg pregabalin
- 10 mg oxycodone
- 75 mg pregabalin followed 1 hour later by 10 mg oxycodone.
- Results
- Subjective
- 75 mg pregabalin
- Increased rating of “feel drug effect,” but that was it.
- 150 mg pregabalin
- Decreased BG scale score of the ARCI and decreased VAS ratings of “in control of body.”
- Increased ratings of difficulty concentrating, heavy or sluggish feeling, and feel drug effect.
- Increased “dreaminess” on the post-session questionnaire.
- No increased ratings of abuse liability-related effects withe either pregabalin dose (e.g. elated, heaving pleasant bodily sensations, like drug, or take again.)
- 10 mg oxycodone
- Increased scores on the LSD scale of the ARCI, increased ratings of “skin itchy,” and increased ratings of “feel drug effect” and “like drug.”
- Ten instances where 75 mg pregabalin or 10 mg oxycodone alone didn’t alter effects, but the combination did.
- Decreased scores on the BG scale of the ARCI
- Increased ratings of “flushing”
- Increased ratings of difficulty concentrating, feel bad, having pleasant bodily sensations, having unpleasant bodily sensations, and light-headed.
- Increased ratings of “coasting (spaced out)” and “headache” on post-session questionnaire.
- 75 mg pregabalin
- Psychomotor and physiological
- Neither drug had any effects on the psychomotor tests.
- 150 mg pregabalin and oxycodone both led to increased exophoria on Maddox Wing Test.
- Oxycodone alone and in combination with 75 mg pregabalin led to decreased pupil size and respiration rate.
- Respiration
- 75 and 150 mg pregabalin led to lower respiration rate, though not dose-responsive.
- Placebo = 12.9
- Pregabalin 75 mg = 10.3
- Pregabalin 150 mg = 10.6
- Oxycodone = 10.5
- Combo = 9.6
- COI: None
- Subjective
(George, 2015) – Positive impact on cardiovascular response induced by intubation
- 80 patients with ASA (American Society of Anesthesiologists) grades 1-2
- Randomized to placebo (40) and pregabalin 150 mg 90-min before surgery (40)
- Results
- Pregabalin led to significant levels of sedation beginning 15 min post-administration
- 26/40 in pregabalin group had RSS of 3 by 30 minutes
- 12/40 in placebo had RSS of 3 by 30 minutes
- Cardiovascular
- Increased HR for both, but a significant difference in favor of pregabalin.
- The difference was significant 1, 3, and 5 minutes after intubation.
- HR returned to normal within 10 min in both groups.
- Also significant attenuation of RPP in the pregabalin group at 1 min and 10 min
- No significant differences in MAP, SBP, or DBP.
- Despite lack of impact on MAP, it did positively impact RPP, which arises from HR and blood pressure.
- Increased HR for both, but a significant difference in favor of pregabalin.
- Pregabalin led to significant levels of sedation beginning 15 min post-administration
- COI: None
Chemistry & Pharmacology
Chemistry
Pregabalin is a member of the gabapentinoid class and a derivative of GABA, an inhibitory neurotransmitter. Another top drug in this category is gabapentin.
It’s also known as 3-isobutyl-GABA, specifically the S isomer of that substance.
Compared to GABA, its 3-position substitution allows for high affinity binding at the alpha-2-delta subunit of voltage-dependent calcium channels. The same substitution allows for drug transport via LAT (system L), an amino acid transport system. And it gets rid of GABA-A and GABA-B activity.
3-isobutyl-GABA was found to be more potent than other 3-alkyl-GABA substances due to it being a substrate for LAT. This property is hypothetically from a similarity to l-leucine, which is carried into the brain via LAT. L-leucine is 2-isobutyl-glycine.
Pharmacology
Despite being a GABA derivative, it lacks GABA-related effects. It’s inactive at GABA-A and GABA-B. It also doesn’t operate through the benzodiazepine site.
Its pharmacology is similar to gabapentin’s, though it’s more potent and has a superior bioavailability.
GABA-B
It didn’t mimic or alter GABA responses with GABA-B receptors in rat hippocampus or recombinant GABA-B receptors expressed in Xenopus oocytes.
Preclinical and clinical studies have shown the activity of pregabalin doesn’t match that of GABA-B agonists. Overall, GABA-B receptors don’t play a role in the mechanism of action.
Inactivity with GABA metabolism and transport
Although it may alter glutamate decarboxylase activity, in vitro results indicate this happens with supratherapetic (millimolar) concentrations. This suggests it’s not an important clinical factor.
(Feng, 2001) – Using rat brain microdialysis, the maximum pregabalin concentration after anticonvulsant doses was found to be 10-50 uM. That’s much lower than the dose connected to enzyme-related activity.
(Errante, 2003) – Neither pregabalin nor gabapentin altered GABA concentrations in rat brain tissues.
(Su, 2005) – Didn’t inhibit GABA transport in vitro, unlike tiagabine (a GABA uptake inhibitor).
A potential impact on GABA transport over time
(Whitworth, 2001)
- Possibly increasing distribution of GABA transporter protein to plasma membrane from intracellular space in cultured neurons with prolonged administration of pregabalin. This would lead to enhancement of GABA uptake.
Primary target: Alpha-2-delta subunit of voltage-dependent calcium channels
While we’re still figuring out the manner by which alpha-2-delta Ca channel inhibition is important, it appears to be the primary target for pregabalin. Along with acutely inhibiting that subunit, it could potentially reduce alpha-2-delta expression over time.
One possible route of effects is through the reduced release of excitatory neurotransmitters, such as glutamate, substance P, and possibly norepinephrine. This would come from reduced influx of calcium at presynaptic neuronal sites.
Its ability to reduce the release of norepinephrine isn’t clear. If it does, it might be secondary to reduced glutamate release. And it may actually increase noradrenergic spinal activity (which would be connected to modulating nociception.)
Along with the aforementioned transmitters, pregabalin could reduce CGRP release, reduce acetylcholine release at the neuromuscular junction, and reduce spinal inhibitory glycine.
Another route involves blocking the action of thrombospondin, a pro-synaptogenic signal operating through alpha-2-delta. That could inhibit excitatory synapse formation.
Distribution
There’s a heterogeneous distribution of the alpha-2-delta subunit throughout the body and the brain. It has a high density in skeletal muscle and the brain, with a low density in the heart, lung, spleen, pancreas, and liver.
Despite binding in non-neuronal tissues like skeletal muscle, pregabalin doesn’t alter muscle contractions from direction stimulation of muscle fibers in vitro (Joshi, 2006). It’s been found to typically lack any notable impact on cardiovascular activity, although some atypical cardiac responses are possible (see: Safety section).
Within the brain, the density is greater in the posterior grey column and in the forebrain, particularly in the superficial layers of the neocortex.
Alpha-2-Delta
Both pregabalin and gabapentin bind specifically to displace labelled gabapentin. Pregabalin has a Ki of 32 nM at recombinant human alpha-2-delta Type 1 proteins (Piechan, 2004).
In mammals, there are at least four related subtypes of the alpha-2-delta protein, each coded by a different gene. Only Types 1 and 2 bind pregabalin with a high affinity.
Binding is similar between alpha-2-delta Type 1 and Type 2 proteins, demonstrating it’s not subtype selective. This makes sense since Types 1 and 2 can be grouped together based on amino acid sequence, while Types 3 and 4 have some differences.
When you use mutations or partial deletions of recombinant alpha-2-delta Type 2 protein, there’s a greatly diminish binding affinity.
In animal models, including the DBA-2 audiogenic seizure model, alpha-2-delta binding is required for efficacy.
Calcium currents
There have been conflicting results regarding its ability to actually reduce neuronal calcium currents. Some studies show a reduction, others don’t.
The difference may come from the expression of the relevant subunits during normal vs. hyperalgesic states. It appears over-expression of the alpha-2-delta subunit could appear when pain is present. Over-expression could also potentially play a role in seizures and even anxiety.
Role for pre-existing disease state
Perhaps there’s a connection between pregabalin’s variable effects in disease/normal states and the NF-kB pathway and inflammation. In vitro studies have found pregabalin can reduce the NF-kB activation caused by substance P.
It’s possible pregabalin is countering activity from NF-kB, which would typically be upregulated in some of the relevant conditions.
Studies
(Lempel, 2016) – Decreases excitability of dentate gyrus and also accelerates the maturation of adult-born granule cells.
- Background
- Evidence supports two potential pathways in which a2-delta calcium channel binding modulates neuronal function.
- Reduction of VDCC currents, either by direct inhibition of VDCC or by reducing surface expression of VDCC.
- Antagonism of interaction by a2-delta and astrocyte-secreted factor thrombospondin, a pro-synaptogenic signal.
- Therefore, it could be exerting an anti-synaptogenic effect that may block abnormal synaptic arrangements following pathological situations.
- Neuropathic pain has indeed been connected to upregulation of a2-delta, thrombospondins, and dorsal horn synaptogenesis.
- Therefore, it could be exerting an anti-synaptogenic effect that may block abnormal synaptic arrangements following pathological situations.
- Studied using mice.
- Results
- Chronic pregabalin led to reduced intrinsic excitability of dentate gyrus granule cells (DGGCs), though only significant for GFP+ neurons.
- Also an increase in spontaneous synaptic transmission.
- So, there are two opposing network activity effects.
- But, the number of Arc positive cells in the dentate gyrus was found to be reduced, suggesting chronic treatment leads to overall reduction in dentate gyrus network activity.
- Evidence for acceleration of maturation of adult-born DGGCs, which fits with earlier evidence (Valente, 2012) showing pregabalin increased differentiation of neural progenitors into neurons.
Pharmacokinetics
Tmax: 1 – 1.5 hours
Cmax: 9.46 ug/mL from a single 300 mg dose
AUC: 66.3 ug x h/mL from a single 300 mg dose
Steady state: Obtained in 24 – 48 hours
Half-life: 6.3 hours
Pregabalin has nearly complete (~90%) oral absorption with linear kinetics. Studies with healthy volunteers have shown predictable kinetics from 75 to 900 mg with low variability.
Only negligible metabolism exists and the drug is eliminated via the renal system. Over 90% of radiolabelled pregabalin (100 mg) was found to be excreted as unchanged drug, with just 0.9% becoming its major metabolite (N-methylated pregabalin).
Because it doesn’t interact with hepatic enzymes, pharmacokinetic interactions with other drugs are largely avoided. This is especially good in populations, like elderly people and epileptics, who may be on multiple medications.
LAT
Like gabapentin, it appears to cross membrane barriers of the gut and BBB via the large amino acid transport system (LAT). That system also transports leucine, isoleucine, and valine.
Pregabalin has been shown to rapidly penetrate the BBB in mice, rats, and monkeys.
However, it appears to also use additional transport systems that allow for more complete non-saturable drug absorption along the small intestine and colon.
In vitro studies suggest pregabalin and gabapentin differ in their uptake by the LAT system in Caco-2 cells. The maximum rate of absorption for pregabalin is about 3-fold higher.
Other factors
Food
No interaction in terms of total drug exposure, though it delays the rate of absorption.
With standard and high-fat meals, the Cmax drops by 25 – 31% and the Tmax is pushed back about an hour.
Renal status
AUC and half-life increase with decreasing renal function, so the dose must be adjusted.
Impaired renal function has been associated with an increased risk of adverse effects.
Race
Based on results from patients with pain or epilepsy, there are no racial differences (among Caucasians, blacks, and Hispanics) for the relationship between daily dose and pregabalin exposure.
Gender
When adjusted for gender-related creatinine clearance differences, there’s no difference.
Drug-drug interactions
No major drug-drug pharmacokinetic interactions have been identified.
None exist with valproate, phenytoin, lamotrigine, topiramate, phenobarbital, carbamazepine, lorazepam, oxycodone, ethanol, oral contraceptives, or various anticonvulsants.
Gabapentin
Coadministration can reduce pregabalin’s Cmax by 18%. But gabapentin’s pharmacokinetics aren’t affected by pregabalin administration.
Studies
(Buvanedran, 2010) – Seeing if it can reach animal analgesic levels acutely in humans
- 9 patients primarily undergoing total knee replacement
- Mean age: 66.6 years
- Mean weight: 90.1 kg
- Given 300 mg pregabalin 1 hour pre-surgery
- PK plasma
- Tmax: 2 hours (2 – 6 hour range) though the sampling only began at 2 hours, so it could be earlier
- Cmax: 6.47 ug/mL
- Half-life: 8.51 hours
- PK CSF
- Tmax: 8 hours (6 – 24 hour range)
- Cmax: 0.41 ug/mL
- Half-life: 21.52 hours
- Interpretation
- CSF drug concentration lags behind plasma significantly.
- Even within 2 hours, the CSF concentration (0.115 ug/mL) is high enough to fit with the antiseizure dose in rats, which was found to be 0.05 ug/mL after a 6 mg/kg dose.
- By 6 hours after the dose, the human CSF level is high enough (0.359 ug/mL) to reduce CNS hypersensitivity based on data from rats, since that dose involves a rodent concentration of 0.334 ug/mL.
- It appears the max pharmacological effect at the spinal level may be achieved around 8 hours after administration.
History
1988
Richard Silverman of Northwestern University asked Dr. Ryszard Andruszkiewicz, a visiting scholar from the Technical University of Gdansk, to synthesize a series of 3-alkyl-GABA and 3-alkyl-glutamate analogs. He then wanted to investigate their inhibition of GABA aminotransferase and glutamate decarboxylase (GAD).
Dr. Andruszkiewicz synthesized fourteen 3-alkyl-GABA analogs (incl. four stereoisomers), 4-methyl-GABA, and seven 3-alkyl-glutamate analogs.
An unexpected finding was that none inhibited GAD. Instead, they activated it. This would lead to an increased rate of glutamate -> GABA, a new mechanism of action.
Silverman immediately responded by having them tested for anticonvulsant activity.
1989
An invention disclosure was submitted to the Northwestern University Technology Transfer Program. Companies were contacted about utilizing these new drugs and their unique mechanism of action.
Only two responded positively: Upjohn and Parke-Davis.
Upjohn decided to exclusively test the “best” compound from the series, while Parke-Davis evaluated all of them.
Because of its decision, Upjohn was left testing the R-isomer of 3-methyl-GABA. When tested on mice, it only had weak anticonvulsant properties, so they discontinued their research into the series.
1990
Parke-Davis called Silverman to invite him to a seminar to discuss the drugs further.
It turned out all of the compounds could reduce tonic extensor seizures in mice at 100 mg/kg, but 3-isobutyl-GABA only required 14.4 mg/kg.
The S-isomer of 3-isobutyl-GABA, which would become “pregabalin,” had turned out to be the best anticonvulsant in the series and one of the best Parke-Davis had ever tested.
A license agreement was signed between Warner-Lambert (parent of Parke-Davis) and Northwestern University. A patent was applied for.
1991
Warner-Lambert and Northwestern signed a patent option agreement.
1992
Animal pharmacokinetic and metabolism studies were carried out, followed by two years of toxicology testing at Parke-Davis.
New syntheses were also developed to obtain larger quantities of (S)-3-isobutyl-GABA.
1995
By the end of 1995, an investigational new drug (IND) was filed with the FDA, opening the way for human testing.
Phase 1 clinical trials then took place over a 2.5-year period.
1996
Until (Gee, 1996) we didn’t really understand how gabapentin or pregabalin were functioning. This study found gabapentin was operating as an alpha-2-delta ligand and the same was then confirmed for pregabalin.
Proteins were solubilized from pig brain membranes and the researchers searched for the fraction that retained labelled gabapentin/pregabalin binding. Once a single protein peak was isolated, it was found to be identical to that previously reported for the alpha-2-delta Type 1 subunit of voltage-dependent calcium channels.
Initially this discovery was surprising. Previously known anticonvulsant sites included voltage-gated sodium channels, GABA-A receptors, GABA degradative enzymes, and GABA uptake transporters.
Meanwhile, calcium channel blockers discovered before the gabapentinoid class didn’t have useful anticonvulsant properties.
The dihydropyridine Ca channel blockers didn’t have significant anticonvulsant activity.
A peptide toxin (agatoxin IVA) selective for blocking P/Q type neuronal Ca channels had potent anticonvulsant activity when injected directly into the brain of DBA/2 audiogenic mice. But it lacked systemic benefits since it would cause death via respiratory collapse due to blocking neuromuscular transmission.
Another toxin selective for N-type Ca channels, ziconotide or omega-Conotoxin MVIIA, had only minor anticonvulsant actions in DBA/2 mice. And they’d cause severe sympathic block systemically.
Given all of this, voltage-dependent Ca channels weren’t considered a go-to spot for anticonvulsant activity in the 1990s.
2000
Pfizer purchased Warner-Lambert, giving it the exclusive rights to continue developing pregabalin.
1999 – 2003
Parke-Davis/Pfizer completed over 100 Phase 2 and Phase 3 clinical trials with 10,000 patients suffering from epilepsy, neuropathic pain, and generalized anxiety disorder.
2002
Although you would have expected Upjohn to have greatly regretted its decision to only test one member of the series, it was also ultimately acquired by Pfizer.
So, even if Upjohn had been the one pursuing a blockbuster drug, Pfizer could have been the one to end up marketing pregabalin.
October 2003
Pfizer filed for a new drug application (NDA) in the US.
2004
It was approved in the EU, initially for neuropathic pain and then as an adjunct therapy for partial seizures.
Pregabalin, under the name Lyrica, entered the European market in September 2004.
2004 – 2005
The FDA approved it December 2004, enabling pregabalin (Lyrica) to enter the US market in September 2005. At the time, it was the first medication approved for both diabetic peripheral neuropathy and postherpetic neuralgia.
It was also placed in Schedule 5. Two core reasons were given for the scheduling:
- The number of individuals reporting euphoria was higher with pregabalin vs. placebo.
- A study involving sedative/hypnotic drug users who were given diazepam (30 mg) or pregabalin (450 mg) showed they could yield similar subjective ratings of “good drug effect,” “high,” and “liking.”
Pregabalin avoided Schedule 4 because it didn’t substitute for benzodiazepines in benzodiazepine-dependent animals and because the positive psychological effects tend to decline with continued use.
2000s
Northwestern initially received a royalty of 6% of net sales from pregabalin and a portion of that was shared with Silverman and Andruszkiewicz.
2006
During its first full year of sales, pregabalin obtained “blockbuster drug” status with $1.2 billion in global sales.
2006
European regulators approved it as a treatment for generalized anxiety disorder.
2007
The FDA approved it for the treatment of fibromyalgia.
2004 – 2013
(Schjerning, 2016) Based on data from Danish nationwide health registries, there was a rise in pregabalin use in Denmark of 7-fold.
2007 – 2013
Northwestern sold the rights to around half of its pregabalin royalties in 2007. It received $700 million from that deal.
Because the money was invested in the endowment and more money came in, the amount grew to around $1.4 billion, making it a large contributor to Northwestern’s endowment.
In 2013, it sold an additional portion of its rights to HealthCare Royalty Partners and the Canada Pension Plan for $290 million.
2010
Pfizer pulled their new drug application for the treatment of generalized anxiety disorder in the US due to a lack of agreement with the FDA. It was, at the time, considering what’s needed to pursue approval for that condition.
2015
Pfizer generated $4.8 billion from the drug.
Legal Status
US (as of April 2017)
Schedule 5
Other countries
Typically it’s a prescription-only substance instead of a controlled drug, though this has different effects in different countries. Often access is restricted, but penalties might not exist for personal possession.
United Kingdom: Schedule 2; Class C
Canada: Prescription-only drug.
Australia: Schedule 4 prescription-only.
Germany: Prescription-only.
Safety
Acute overdose
CNS depressant effects are the most significant elements of an overdose, along with dizziness. When taking more than a gram, you’re likely to experience significant sedation and impaired motor control. Coma is a possibility with certain doses. Cardiac issues are another hypothetical risk.
There’s unclear evidence regarding the potential for respiratory depression. Even in cases with multiple gram overdoses, cardiac and respiratory stability has been present, but the risk shouldn’t be ruled out.
Even very significant overdoses, as long as it’s not combined with other drugs, are unlikely to result in a fatal response. An 8 gram overdose was reported during clinical trials without death or serious adverse effects. Another 8+ gram non-fatal overdose is also described below.
However, there’s still some potential for an acute overdose to be deadly, especially in those with a preexisting cardiovascular issue or impaired renal function.
Adverse effects (medical settings)
Most of the adverse effects in medical settings are mild and CNS-related. At doses up to 600 mg/d, the negatives are usually tolerable and the drug has a high safety level.
However, there are rare atypical responses. Among those are some hematological reactions, including neutropenia, thrombocytopenia, and leukopenia.
Allergic reactions are also possible, with hypersensitivity skin rashes occurring rarely.
Other rare responses are cardiovascular issues and hepatotoxicity, with the former appearing to be somewhat more common than the latter.
Cardiovascular issues and heart failure
There might be a higher rate of cardiovascular issues among pregabalin patients vs. gabapentin patients. There’s a growing number of cases suggestive of a real link.
The difference between pregabalin and gabapentin may have to do with differences in Ca channel antagonism.
It’s been suggested that some of the edema seen with pregabalin could be heart failure-related.
L-type Ca channel blockers can lead to vasodilation of mesenteric resistance arteries. This uncompensated arteriolar vasodilation can lead to intracapillary hydrostatic pressure increase, thereby exuding fluid into the interstitium, causing peripheral edema. A similar situation may rarely occur with pregabalin, but more evidence is needed.
More caution in prescribing and use should exist when someone has a history of renal and/or cardiovascular disease.
Reviews
(French database)
- The Fernand-Widal Regional Pharmacovigilance Centre in Paris analyzed 41 reports of cardiac adverse effects attributed to Pregabalin
- They had a median age of 74 years and were mainly using it for neuropathic pain.
- 25 cases had arrhythmia or conduction disorders (bradycardia, tachycardia, atrioventricular block, atrial fibrillation).
- 13 cases of heart failure
- 5 cases of palpitations
- 1 case of myocardial infarction
- 26/41 cases were considered severe
- Often the dose was under 150 mg and the onset was quick
- Average dose of 100 mg
- Average onset of 9 days
- Recovery
- Status known in 35 cases
- 30 patients recovered, 3 were “improving,” and 2 died (one from cardiac decompensation in a 77-year-old female and one from myocardial infarction in a 58-year-old male)
- The latter death also involved gemcitabine and cisplatin.
- 32/41 cases involved people with a history of cardiac disorders.
Cases
(Page, 2008) – 3 cases suggestive of negative effects in those with heart failure
- Case 1
- 74-year-old female with nonischemic cardiomyopathy and class 3 heart failure; ejection fraction of 55%
- Stable for 3 months with medications
- Initially received pregabalin 150 mg/d for a pinched nerve
- Over following 3 days: shortness of breath, confusion, and weight gain
- Day 7 of pregabalin: unresponsive and unconscious at home
- Arrived at ED
- Obtunded, dyspneic, hypotensive, and increased serum creatinine, lower extremity edema.
- Given intubation, IV furosemide, nitroglycerin, nitroprusside, and dopamine.
- Pregabalin stopped and symptoms improved.
- Case 2
- 48-year-old male with nonischemic dilated cardiomyopathy and class 2 heart failure; ejection fraction of 45%
- Stable for 14 months with medications
- Pregabalin 150 mg/d given for chronic neuropathic pain
- 3 days later: fatigue, shortness of breath, peripheral edema, 2.2 kg of weight gain
- Within 1 month of stopping pregabalin: resolution of additional heart failure symptoms and weight gain
- Case 3
- 61-year-old male with ischemic cardiomyopathy and class 2 heart failure; ejection fraction of 45%
- Stable for 6 months with medication
- Pregabalin 100 mg/d given for neuropathic pain
- Within 2 months of starting: fluid retention, shortness of breath, abdominal bloating, ankle edema, and weight gain.
- Symptoms escalated over 3 months
- Admitted to hospital for worsening heart failure
- Symptoms suggestive of volume overload with a 6 kg weight gain.
- ECG showed worsening left ventricular function and severe mitral and tricuspid regurgitation.
- Pregabalin stopped
- Gabapentin replaced it and no worsening of status was seen.
Hepatotoxicity
Some cases exist, but they’re very uncommon. It may be more problematic when there’s preexisting liver injury. So far there’s no good evidence for a dose-dependency.
(Sendra, 2011)
- 59-year-old initially presented to hospital with persistent fever for 3 days
- He had a history of allogenic transplant 11 months prior.
- Lab data initially showed increased leukocytes, C-reactive protein, lactate dehydrogenase
- And slightly elevated AST and ALT.
- After 6 days of fever with no clinical focus, oral levofloxacin 500 mg was started.
- Patient described neuropathic pain in one foot; received 25 mg pregabalin twice daily
- Four days after starting levofloxacin, the patient had no fever and had clear clinical improvement
- Antibiotic therapy was continued for another 5 days.
- 14 days after starting pregabalin
- Reported left ankle edema
- Significant rise in liver enzymes
- AST – 907
- ALT – 1582
- GGT – 510
- Also higher total bilirubin, direct bilirubin, creatinine, and others.
- Pregabalin was stopped given it was the only fully new medication.
- Decline in liver enzyme levels seen at 2 days post-pregabalin and continued decline by 7 days.
- By a 4-month follow-up
- Completely normal liver function test results.
- Interpretation
- Likely an idiosyncratic reaction to pregabalin with potential underlying liver injury as another factor.
(Dogan, 2011)
- 28-year-old female
- Presented with a 3 – 4 day history of jaundice.
- Four months before presentation, was prescribed pregabalin 300 mg/d.
- AST and ALT levels at presentation were 26x above the upper limit and total bilirubin was 16.3 mg/dl.
- Pregabalin stopped after admission because of elevated liver function tests.
- Two months later, all tests were normal.
- Pregabalin was considered the cause, though there were two other instances leading to higher liver enzyme levels
- Both were courses of IV glucocorticoid pulse. Although the first is known to have increased levels, they were normal again by the second course, so it’s hard to view it as non-pregabalin related.
Suicidality
In 2008, the FDA issued a warning that antiepileptics, including pregabalin, may raise the risk of suicide. Although this warning has been criticized and there’s still not a ton of data, it’s worth keeping in mind. The risk of suicidal ideation may be somewhat increased as a result of the substance.
Physical dependence & tolerance
Tolerance and withdrawal aren’t major issues with the drug at medical doses. Though a short-term taper is still recommended for patients.
In other settings where the dosing is higher, withdrawal can be quite unpleasant. It can include nausea, insomnia, headache, sweating, diarrhea, anxiety, and a sick flu-like feeling. This kind of withdrawal is easiest to avoid simply by using common doses and not taking it often.
Tolerance is said to build pretty fast in recreational settings. Within a few uses, the effects will decline. This leads to some people eventually taking well over 1 gram per day. That’s when the risk of withdrawal is much higher.
Fatality reviews
(Lottner-Nau, 2013) – Reviewing postmortem toxicology from 2010 to 2012 in Germany
- 4200 autopsies and 982 toxicological analyses performed
- Pregabalin detected in 4.4% (n=43)
- Median concentration in whole blood was 5.18 mg/L (range of 0.04 to 23.8 mg/L)
- One or more drugs was found in every case
- Most popular: opioids, benzodiazepines, alcohol, and antidepressants.
- For opioids, the top ones were: fentanyl, methadone, morphine, codeine.
Problematic responses
(Lee, 2011) – Two cases of negative neurological symptoms in chronic kidney disease patients
- Case 1
- 67-year-old male
- Pregabalin started 2 weeks before presentation at 300 mg/d
- Upped to 450 mg/d 7 days before presentation
- Presented with deep drowsy state, generalized myoclonic jerk, aphasia, and dysarthria.
- No brain lesion detected, nor lab abnormalities.
- Pregabalin blamed; withdrawn
- With withdrawal of pregabalin and additional hemodialysis sessions, he became mentally alert and the other symptoms disappeared entirely.
- Case 2
- 43-year-old male
- Diagnosed with fibromyalgia and given pregabalin
- After receiving 75 mg/d for 2 days
- Developed drowsy mental state and myoclonus of both upper extremities.
- Pregabalin withdrawn
- All symptoms resolved
Non-fatal overdoses
(Wood, 2010)
- 54-year-old male
- Reported to ED after using ~8.4 grams of pregabalin
- At presentation
- Alert with GCS of 15
- Cardiovascularly stable: HR of 99, BP of 127/60, 37.0°C temp, and 20 respiratory rate.
- Given activated charcoal since he was presenting under an hour after ingestion.
- 3 hours post-ingestion
- Deterioration to GCS of 4/15
- Cardiovascularly stable with HR of 99 and BP of 134/85
- ECG showed borderline sinus tachycardia, but QRS and QTc were normal.
- Intubated for airway protection and mechanically ventilated.
- GCS improved over the next 24 hours while remaining cardiovascularly stable, enabling extubation 26 hours after admission to ICU.
- Toxicology
- Serum at 3 hours post-ingestion: 66.5 mg/L
- Serum at 7 hours: 51.2 mg/L
- Serum at 27 hours: 15.2 mg/L
References
(2017) Pregabalin for the treatment of neuropathic pain: a narrative review for primary care providers.
(2016) Pregabalin for decreasing abdominal pain in people with chronic pancreatitis
(2016) Pregabalin for the Treatment of Drug and Alcohol Withdrawal Symptoms: A Comprehensive Review
(2015) Premedication dilemmas, is Pregabalin the answer?
(2014) Pregabalin add-on for drug-resistant partial epilepsy.
(2014) A review of the effects of pregabalin on sleep disturbance across multiple clinical conditions.
(2013) Pregabalin for the treatment of generalized anxiety disorder: an update
(2013) Antiepileptic drugs for neuropathic pain and fibromyalgia – an overview of Cochrane reviews.
(2013) Risk of heart failure and edema associated with the use of pregabalin: a systematic review
(2013) Abuse of pregabalin – results of the postmortem toxicology from 2010 to 2012
(2013) Bad medicine: gabapentin and pregabalin
(2013) Pregabalin effective for the prevention of chronic postsurgical pain: really?
(2013) Pregabalin for the treatment of generalized anxiety disorder: an update.
(2012) Efficacy and safety of pregabalin in the treatment of alcohol and benzodiazepine dependence.
(2012) Pregabalin monotherapy for epilepsy.
(2012) Pregabalin in clinical psychiatry and addiction: pros and cons.
(2011) Efficacy of pregabalin in acute postoperative pain: a meta-analysis.
(2011) Earlier discovery of pregabalin’s dependence potential might have been possible
(2011) Pregabalin-induced hepatotoxicity.
(2011) Pregabalin-induced hepatotoxicity
(2011) Possible heart failure associated with pregabalin use: case report.
(2011) Two cases of pregabalin neurotoxicity in chronic kidney disease patients
(2010) Significant pregabalin toxicity managed with supportive care alone.
(2010) A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin.
(2009) Mechanisms of analgesia by gabapentin and pregabalin – Calcium channel α2-δ [Cavα2-δ] ligands
(2008) Pregabalin: a review of its use in fibromyalgia.
(2008) From basic science to blockbuster drug: the discovery of Lyrica.
(2006) Pregabalin: From molecule to medicine.
(2006) The mechanisms of action of gabapentin and pregabalin.
(2005) Pregabalin: a new neuromodulator with broad therapeutic indications.
(2005) Pregabalin
(2004) Pregabalin pharmacology and its relevance to clinical practice.
(2003) Pharmacokinetics of pregabalin in subjects with various degrees of renal function.