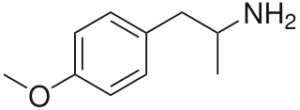
PMA is a stimulant in the amphetamine class. It has rarely been used intentionally, but it’s been an adulterant in the MDMA/MDA market for decades. Due to its toxicity, which is relevant even at relatively low doses, hundreds of deaths have likely come from its use.
The initial effects of a common dose resemble MDMA in some ways, but higher doses don’t develop into an entactogen experience. Instead, the primary effects become agitation, confusion, tachycardia, hyperthermia, and hypertension.
It exerts its effects by releasing and inhibiting the reuptake of serotonin. Additionally, it’s an MAO-A inhibitor, which contributes to the negative effects.
PMA = para-methoxyamphetamine; paramethoxyamphetamine; 4-methoxyamphetamine, 4-MA, P-Methoxyamphetamine, 1-p-Methoxyphenyl-2-propylamine
PubChem: 31721
Molecular formula: C10H15NO
Molecular weight: 165.236 g/mol
IUPAC: 1-(4-methoxyphenyl)propan-2-amine
Dose
Oral
Light: 30 – 40 mg
Common: 40 – 50 mg
Strong: 50 – 60+ mg
Timeline
Oral (tentative)
Total: 2 – 4 hours
Onset: 01:00 – 02:00 (some reports claim over 2 hours)
Experience Reports
Supplementary Videos
References
(2016) A fatal case of paramethoxyamphetamine poisoning and its detection in hair.
(2012) Severe paramethoxymethamphetamine (PMMA) and paramethoxyamphetamine (PMA) outbreak in Israel.
(2008) Fatal paramethoxy-amphetamine (PMA) poisoning in the Australian Capital Territory
(2003) Report on the risk assessment of PMMA in the framework of the joint action on new synthetic drugs
(2003) A fatal paramethoxymethamphetamine intoxication.
(2003) Paramethoxyamphetamine (PMA) poisoning; a ‘party drug’ with lethal effects.
(2003) Anise oil as para-methoxyamphetamine (PMA) precursor.
(2002) Death and paramethoxyamphetamine – an evolving problem. [Response]
(2002) Death and paramethoxyamphetamine — an evolving problem.
(2001) Three cases of fatal paramethoxyamphetamine overdose.
(2001) Fatalities Caused by the MDMA-related Drug Paramethoxyamphetamine (PMA)
(2001) Poisoning with the recreational drug paramethoxyamphetamine (“death”).
(2000) The hallucinogen PMA: Dancing with death
(1998) Hyperpyrexia associated with fatal paramethoxyamphetamine (PMA) abuse.
(1998) Recent paramethoxyamphetamine deaths.
(1984) The stimulus properties of para-methoxyamphetamine: a nonessential serotonergic component.
(1974) PMA deaths in Ontario
(1971) Effect of para-methoxyamphetamine on catecholamine metabolism in the mouse brain.