Codeine is an opioid found naturally in Papaver somniferum, the opium poppy. It’s somewhat misleading to call it an opioid since codeine primarily functions as a prodrug, offering little of its own activity. Morphine seems to be its primary active metabolite.
The drug is typically a controlled substance, yet it may also be available over-the-counter (OTC) in certain combinations.
Its name comes from the Greek word “kodeia,” which means “poppy head.”
Medically, codeine is used as an analgesic, antitussive, and antidiarrheal. It’s also taken recreationally, often at higher doses.
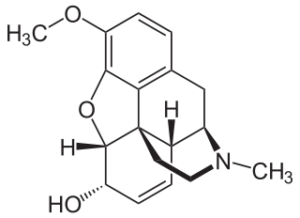
Codeine = Methylmorphine; 3-methylmorphine
PubChem: 5284371
Molecular formula: C18H21NO3
Molecular weight: 299.37 g/mol
IUPAC: (4R,4aR,7S,7aR,12bS)-9-methoxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-7-ol
Contents
Dose
Oral (medical)
- 15 – 60 mg every 4 – 6 hours
Oral (nonmedical)
- Light: 50 – 100 mg
- Common: 100 – 150 mg
- Strong: 150 – 200 mg
You should use a light dose before attempting any larger amounts. It’s wise to avoid going above the common range.
Timeline
Oral
Total: 3 – 6 hours
Onset: 00:30 – 00:45
Experience Reports
Effects
Positives
- Analgesia
- Sedation
- Euphoria
- Anxiolysis
- Physical euphoria
- Dreaminess
- Contentment
Negatives
- Respiratory depression
- Drowsiness
- Depression
- Nausea
- Vomiting
- GI motility reduction/constipation
- Bradycardia
- Dysphoria
Medical
The drug is mainly used as an analgesic, antitussive, and antidiarrheal.
Analgesic (pain relief)
Evidence supports the existence of codeine’s analgesic effects in minor to moderate pain, yet the drug usually doesn’t make a lot of sense compared to other analgesics. It’s primarily a prodrug, with its most relevant metabolite being morphine. In which case, there’s a good argument in favor of simply using morphine itself. Using codeine means administering an unknown dose of morphine since that metabolic pathway varies between people.
If people don’t generate enough morphine, they may receive little to no benefit. And if they generate too much, severe negative effects could be more likely.
The evidence also suggests NSAIDs, paracetamol, and other opioids are superior to codeine in nearly every case. People can often receive adequate pain relief just with NSAIDs and/or paracetamol.
When looking at codeine from a purely efficacy-focused perspective, it doesn’t make much sense. But because of its widespread availability and unjustifiably good reputation, it’s one of the world’s most popular analgesics.
In cases where it works, the drug can dull pain sensation to the extent that it becomes bearable and sometimes barely noticeable.
Pediatric
Some medical institutions have switched to only giving codeine to children 12-and-older. It has occasionally caused severe respiratory depression and death at medical doses in young children.
There’s little reason to give it as a first-line analgesic. NSAIDs and/or paracetamol are frequently adequate.
It is specifically contraindicated in the postoperative period following tonsillectomy and adenoidectomy for obstructive sleep apnea.
Despite it not being a great first-line drug, codeine is commonly prescribed to children postoperatively.
Papers
(Cochrane Database Review, 1998) – Comparing single doses of paracetamol alone vs. with codeine
- NNT used for comparison (for 50% analgesia for 4-6 h vs. placebo)
- Just limited data from two trials and 127 total patients.
- Postoperative pain
- Paracetamol 1000 mg had an NNT of 4.6
- (Cochrane, 2000) – The NNT for codeine 60 mg alone is 17
- Paracetamol 1000 mg + codeine 60 mg had a NNT of 1.9
(Derry, 2010) – Cochrane review of single dose codeine for acute postoperative pain in adults
- 35 studies yielding 1223 people given codeine 60 mg, 27 given codeine 90 mg, and 1252 given placebo.
- Combining all types of surgery (33 studies, 2411 participants)
- Codeine 60 mg had an NNT (50% pain relief over 4-6 hours) of 12 (8.4 to 18) compared with placebo.
- At least 50% pain relief was achieved by 26% on codeine and 17% on placebo.
- Only dental surgery (15 studies, 1146 participants)
- NNT of 21 (12 to 96)
- Only other types of surgery (18 studies, 1265 participants)
- NNT of 6.8 (4.6 to 13)
- Prevent use of rescue medication within 4-6 hours (11 studies, 765 participants, mostly non-dental)
- NNT of 11 (6.3 to 50)
- More participants had adverse events within codeine 60 mg than placebo, but the difference was non-significant.
- Conclusion
- Codeine 60 mg offers good analgesia to few people. It doesn’t compare favorably to paracetamol, NSAIDs, or combos with codeine, especially after dental surgery.
- Other common analgesics, either alone or with codeine, offer superior pain relief.
(Toms, 2016) – Cochrane review of paracetamol with codeine for postoperative pain in adults
- 26 studies with 2295 participants
- Significant dose-response was seen for NNT (at least 50% pain relief over 4-6 hours)
- 2.2 for 800-1000 mg paracetamol with 60 mg codeine
- 3.9 for 60-650 mg paracetamol with 60 mg codeine
- 6.9 for 300 mg paracetamol with 30 mg codeine
- Time to use rescue medication
- 4+ hours with the combo vs. 2 hours with placebo
- NNT to prevent remedication
- 5.6 for 600 mg paracetamol and 60 mg codeine over 4-6 hours
- Adverse events increased mainly of mild/moderate severity.
- 14 studies with 926 participants compared paracetamol/codeine vs. paracetamol alone
- Codeine increased the proportion receiving 50% pain relief by 10-15% and increased time to rescue medication by 1 hour. It also reduced the proportion needing rescue medication by about 15%.
- Adverse events didn’t significantly differ between groups.
- Interpretation
- Confirms paracetamol with codeine offers clinically useful analgesia for a large portion of patients. Around 50% will receive significant pain management, compared with under 20% with placebo.
- Codeine can somewhat improve upon paracetamol alone.
(Smith, 2001) – Comparing paracetamol alone vs. with codeine
- Found the NNT for paracetamol 1000 mg + codeine 60 mg was 2.2 (indicating superior efficacy to paracetamol alone)
(Quiding, 1984) – Single-dose studies may underestimate the efficacy of codeine.
- Showed single-dose studies could improperly describe the full analgesic activity of codeine in patient populations receiving multiple doses.
- Pain from third molar extraction
- Pronounced improvement with the 2nd dose of codeine 60 mg compared to the first.
(Straube, 2016) – Cochrane review of its use, alone or with paracetamol, for cancer pain
- 15 studies – 721 adult participants w/ cancer pain from diverse types of malignancy.
- Results couldn’t be combined due to them being reported differently.
- All but 1 of the studies had a high bias risk due to small study size.
- 12 studies involved codeine alone, while 3 combined with it with paracetamol.
- Dose: 30 to 120 mg
- 11 reported treatment group mean measures of pain intensity or pain relief
- Based on these, codeine or codeine + paracetamol were superior to placebo and equivalent to active comparators.
- Negatives
- Nausea, vomiting, and constipation were common.
- Drowsiness and dizziness also occurred.
- Summary: Based on limited low-quality evidence, codeine is superior to placebo in the treatment of cancer pain.
(Po, 1998) – Meta-analysis of ibuprofen’s efficacy alone or in combination without caffeine or codeine
- 39 reports on ibuprofen vs. placebo; 8 with ibuprofen + codeine vs. placebo (375 patients vs. 324)
- Results
- Indirect comparisons
- Codeine failed to significantly enhance the analgesic effect of ibuprofen.
- Head-to-head comparisons
- 400 mg ibuprofen + 60 mg codeine was significantly superior to 400 mg ibuprofen alone.
- A separate crossover study showed codeine 20 mg added to the analgesia of ibuprofen 400 mg, whereas codeine 15 mg didn’t improve ibuprofen 200 mg.
- Negatives
- Only drowsiness was significantly increased with ibuprofen vs. placebo.
- Risk of drowsiness was 2x higher with ibuprofen and 4x higher with ibuprofen/codeine.
- Indirect comparisons
(Moore, 1997) – Systematic review of paracetamol alone vs. with codeine
- Paracetamol plus codeine vs. placebo (19 trials with 631 active patients and 573 placebo patients)
- Mean response rate
- 54% for paracetamol 600/650 mg + codeine 60 mg vs. 21% for placebo
- NNT
- Paracetamol 300 mg + codeine 30 mg had an NNT compared with placebo (at least 50% pain relief) of 5.3
- Paracetmaol 600/650 mg + codeine 60 mg had an NNT of 3.1
- Mean response rate
- Paracetamol plus codeine vs. paracetamol alone (13 trials with 435 active patients and 439 placebo patients)
- 10 trials in oral surgery; 3 in postsurgical pain
- NNT
- Addition of codeine 60 mg to all doses of paracetamol in single dose administration was 9.1
- Mean response rate
- Adding codeine 60 mg to paracetamol led to a 55% response rate, compared to a 43% rate with paracetamol alone.
- The lack of overlap in the 95% confidence interval of the NNT for paracetamol 600/650 mg + codeine 60 mg (2.6-3.8) and that of paracetamol 600/650 mg alone (4.1-6.9) indicates a significant beneficial effect of adding codeine to paracetamol therapy.
(Drendel, 2009) and (Friday, 2009)
- Comparing ibuprofen 10 mg/kg to paracetamol/codeine (with codeine at 1 mg/kg/dose)
- Ibuprofen was at least as effective as paracetamol-codeine for outpatient analgesia in children with acute traumatic extremity pain. No significant difference in analgesia, but children given ibuprofen had better functional outcomes. And children aged 4-18 years given ibuprofen had significantly fewer adverse effects, and both children and parents were more satisfied with ibuprofen.
(Lasagna, 1954)
- 30 mg of SC codeine was inferior to 10 mg morphine for postoperative pain.
- 60 mg of SC codeine was close to 10 mg morphine, but still not the same.
- And even 120 mg of SC codeine failed to equal the performance of morphine.
(Cooper, 1982) – Analgesic efficacy of ibuprofen-codeine vs other options
- Double-blind
- Groups
- 38 – ibuprofen 400 mg
- 41 – ibuprofen 400 mg + codeine 60 mg
- 38 – aspirin 650 mg
- 45 – aspirin 650 mg + codeine 60 mg
- 41- codeine 60 mg
- 46 – placebo
- Patients selected from population scheduled for surgical removal of 1-4 impacted third molars.
- When the patients’ postsurgical pain reached moderate to severe intensity, they self-administered the study medication.
- Results
- Sum of Pain Intensity Differences (SPID) AUC
- Ibuprofein-codeine had the greatest efficacy
- Ibuprofen 400 mg was significantly superior to both aspirin 650 mg and codeine 60 mg
- Ibuprofen 400 mg was also non-significantly superior to aspirin-codeine
- Ibuprofein-codeine was significantly superior to aspirin-codeine
- Total Pain Relief (TOTPAR) AUC
- Ibuprofen was superior to both the aspirin and codeine treatments
- Ibuprofen-codeine was superior to aspirin-codeine
- Overall Impression
- Aspirin-codeine, ibuprofein-codeine, and ibuprofen were superior to aspirin alone and codeine alone
- Ibuprofein-codeine had the highest mean score
- Side effects
- 66 patients reported a total of 71 side effects
- No significant differences among the treatment groups for the frequency of side effects.
- Most common was drowsiness (38 reports)
- 13 from ibuprofen-codeine
- 5 from aspirin-codeine
- 8 from ibuprofen
- 5 from aspirin and codeine groups
- 2 from placebo
- Next most common was nausea (8 reports)
- 4 from ibuprofen-codeine
- Only 1 euphoria report, arising from the aspirin+codeine combo
- 66 patients reported a total of 71 side effects
- Interpretation
- 6/7 efficacy measures showed ibuprofen-codeine was best, followed by ibuprofen, aspirin+codeine, aspirin, codeine, and placebo.
- Sum of Pain Intensity Differences (SPID) AUC
(Charney, 2008) – Oxycodone vs. codeine for pain associated with forearm fracture in children
- 107 children aged 4-17
- 51 got oxycodone; 56 got codeine
- Received either 0.2 mg/kg oxycodone (max of 15 mg) or 2 mg/kg codeine (max of 120 mg)
- Completed questionnaire about pain status and adverse effects at 30-min intervals
- Results
- Both groups experienced significant pain reduction from baseline. Those receiving oxycodone had significantly greater pain reduction vs. codeine.
- Adverse
- No significant differences with nausea.
- Itching was less common in the oxycodone group.
- All other adverse effects were similar.
- 2 codeine patients had urticaria, which resolved after diphenhydramine.
- Satisfaction
- Parents in both groups reported high levels of satisfaction and wished to have the medication given again under similar circumstances.
Alternatives for treating pain
NSAIDs have been discouraged in the postoperative setting due to their impact on platelet function. Yet studies indicate they don’t raise the risk of bleeding that necessitates a return to the operating room.
Paracetamol dosing recommendations have declined in recent years. This is attributed to concerns about hepatotoxicity arising from a metabolite. That adverse effect is rare at common doses.
Antitussive (cough suppression)
It doesn’t appear effective in the majority of cough cases. Evidence for its efficacy is lacking, while multiple trials have found it’s no better than placebo for cough associated with an upper respiratory infection (URI), like the common cold, or for cough associated with COPD.
Antitussive in children
While it is still used for cough in children, it’s not recommended. There’s a lack of proven efficacy and safety risks make it an unwise choice.
- April 2015 – European Medicines Agency said codeine must not be used for this purpose in children under 12.
- July 2015 – FDA drug safety communication stated, based on an investigation of the risks, that it shouldn’t be used as an antitussive in children under 18 due to potential for serious problems, including slowed or difficult breathing.
- December 2015 – FDA advisory panel met and overwhelmingly recommended the use of codeine for cough be contraindicated in all children under 18-years-old.
General
Even though it’s widely used for cough, such as with the common cold, it’s not actually recommended. The American College of Chest Physicians doesn’t recommend codeine or other opioids for cold-related cough. And a Cochrane review of two RCTs found it was no more effective than placebo.
Animal models have shown efficacy, as have some human trials involving induced and chronic cough. But the prominent usage of codeine for cough associated with URI can’t be justified by the evidence.
The lack of impact in URI-related cough vs. induced or chronic cough may arise from the existence of multiple cough control pathways. URI-related coughs could invlve a “voluntary” pathway, while the others involve a reflex pathway. Codeine doesn’t seem to have any notable effect on the voluntary pathway.
Trials showing a lack of impact in COPD suggest it may also be ineffective for chronic cough stemming from lower airway disorders.
Cough associated with URI
Support for its efficacy is very weak, coming from older and poorer trials.
(Glick, 1963) – Compared codeine to caramiphen in patients with cough associated with acute URI
- After 3 days, 7/12 with codeine reported satisfactory results vs. 9/12 with caramiphen.
Better trials have shown a lack of efficacy.
(Eccles, 1992) – Double-blind placebo controlled trial with 91 patients
- Non-productive cough as a symptom for 6-96 hours before enrollment
- Lab phase (observed coughing over 3 h) and home phase (reports for 4 days)
- Lab phase
- Significant reduction in cough in both groups, but no significant difference between groups at any time point.
- Home phase
- Significant reduction in cough in both groups (codeine 30 mg at 4x per day vs. placebo), with no significant difference at any time.
(Taylor, 1993) – Double-blind placebo controlled trial of codeine and DXM in night time cough in children associated with URI
- 57 patients
- Given one of 3 treatments
- Guaifenesin 100 mg and DXM 15 mg
- Guaifenesin 100 mg and codeine 10 mg
- Placebo
- Results
- Symptoms improved in each group on each day, with no significant differences between groups on any day.
- Suggests neither codeine nor DXM is actually superior to placebo in alleviating night cough from URI.
(Freestone, 1997)
- 82 subjects with cough from acute URI
- Cough sound-pressure levels
- Codeine group and placebo group both had significant declines in mean CSPL, with no significant differences between the groups at start or 90 min post-treatment.
- Cough frequency
- Codeine group and placebo group both had significant declines in frequency, with no significant differences between the groups at start or 90 min post-treatment.
- Subjective scores
- Codeine group and placebo group both had significant declines in frequency, with no significant differences between the groups at start or 90 min post-treatment.
- Interpretation
- Codeine is no more effective than placebo in reducing cough associated with acute URI.
Narcolepsy
Some case reports and trials indicate codeine could help people with narcolepsy. If it works, it may reduce daytime sleepiness.
(Harper, 1981)
- 55-year-old narcoleptic
- Excessive daytime sleepiness (EDS) symptoms were relieved by either codeine or pentazocine, which were given for pain and diarrhea from Crohn’s disease.
(Fry, 1986) – Clinical reports and some trials
- Study 1 – Open trial
- 5 patients
- Given 30 mg ~30 min after awakening and then every 3-4 h during the day until a total of 5 doses of codeine were taken.
- After receiving codeine for 2+ weeks, the subjects went repeated the maintenance of wakefulness test (MWT).
- Results
- Before codeine, all subjects had sleep latencies within pathological daytime sleepiness.
- In the first 4 subjects, the tendency was to increase the MWT, but the tests tended to remain pathological.
- In the 5th subject, MWT changed to being in the range of normal daytime sleepiness.
- As a group, the overall increase in MWT didn’t reach significance.
- Subjective
- Yet all subjects had a dramatic clinical improvement.
- 3/5 reported a decline in the frequency of cataplectic attack.
- 2 who had reported frequent amnestic periods and automatic behaviors reported a complete resolution.
- 4/5 subjects preferred codeine over prior stimulant medications because of its effectiveness and lack of side effects.
- Only side effect was constipation, which was fairly well controlled by stool softeners and mild laxatives.
- Study 2 – Double-blind
- 8 patients
- Each subject received placebo for 1 week and codeine for 1 week. The order was random.
- Results
- Analysis of nocturnal sleep parameters and the nine daytime levels of alertness and sleep failed to show any significant effect.
- However, analysis of patient diary data showed a significant reduction in the number of daily naps from 3.2 to 1.9.
- Clinical experience
- 27 diagnosed narcoleptic patients were treated with codeine
- Results
- 18/27 continued to take codeine and were taking it for 10-33 months
- 8/9 who stopped did so within 6 weeks
- 7 – Ineffectiveness
- 1 – Worsening of depression
- 1 – Allergic rash
- Among the continuing 18, 8 had no side effects, 7 had mild constipation, 2 had severe constipation, and 1 had “slowed thinking.”
- Dose: 90 to 180 mg/d in divided doses for 14 patients and 30 to 90 mg/d for 1-3 days per week in the other 4
- All 18 said codeine was definitely effective for daytime sleepiness
- The 4 not taking it daily had mild EDS and only took codeine during their worst periods or when unable to take regular naps. They reported codeine taken as needed dependably reduced EDS.
- All of the 14 taking it daily are employed and/or attending college. They reported an improvement in daily performance due to a reduction in EDS and amnestic episodes.
- Of the 18, 14 had previously used stimulants.
- 8 preferred codeine due to stimulant side effects, 3 due to stimulants being less effective, 3 for both reasons.
- Interpretation
- Subjective reports indicate codeine is effective for EWS in narcolepsy.
- However, measures like the MSLT and MWT fail to corroborate that subjective efficacy. But this has been seen in multiple instances with other typical narcolepsy treatments (subjective reports don’t align with the standard tests).
Medical combos
Promethazine
This combination is used in cough and cold preparations, where it’s recommended for alleviating cold or allergy symptoms like runny nose, sneezing, and cough. It’s primarily used as a cough syrup. Promethazine doesn’t seem to offer any notable cough suppressant properties, yet it could raise the risk of negatives. Promethazine likely helps with allergy symptoms, but the combination doesn’t typically make sense for cough.
Paracetamol and NSAIDs
Codeine with NSAIDs or paracetamol can increase analgesia.
Negatives in medical settings
Constipation is one of the most problematic negatives. It frequently necessitates the use of laxatives.
Other negatives include drowsiness, dizziness, depression, nausea, and vomiting.
A Cochrane review found it didn’t significantly raise the risk of nausea or vomiting vs. paracetamol given alone. Though it did raise the risk of drowsiness and dizziness.
Respiration
At or near medical doses, codeine may begin to impact respiration, albeit not in a dangerous way. Some tests have found no effect at medical doses, however.
(Pleuvry, 1980) – Comparing the effects of codeine and promethazine on males vs. females
- 10 healthy volunteers; 5 of each sex + 6 additional smokers
- Given placebo, 60 mg codeine, 50 mg promethazeine, or both
- HR was not significantly altered by any drug treatment. Only a fall in diastolic blood pressure was significant after promethazine.
- Respiration (mean minute volume, tidal volume, end tidal PCO2)
- Drug treatments didn’t significantly alter those measurements in male volunteers, but in female subjects, both promethazine and codeine separately caused significant changes.
- Promethazine reduced tidal volume and minute volume 1 and 2 h post-ingestion. Those changes were significant.
- Codeine on its own didn’t raise end tidal PCo2 in females.
- Drug treatments didn’t significantly alter those measurements in male volunteers, but in female subjects, both promethazine and codeine separately caused significant changes.
- Ventilatory response to CO2
- No slope differences between males and females. But smokers had a much steeper ventilary response to CO2 than non-smokers.
- Effect on displacement of the ventilary response to CO2
- Codeine had no significant impact in males, but displaced the response relationship to the right in females. This is consistent with the finding of an increased end tidal PCO2 in females breathing air.
- Males given codeine and promethazine exhibited a rightward displacement of the CO2 response that was greater than with codeine alone or placebo. In contrast, females showed a similar but slightly smaller displacement of the response, though the reduction wasn’t significant.
- Promethazine alone led to no significant change in CO2 response.
- Subjective
- Promethazine (alone and in combo) caused drowsiness in some and led to sleeping in some.
- Extreme pallor was seen in 4 subjects given promethazeine. This was absent when combined with codeine.
Impact on driving
(Linnoila, 1973) – Simulated driving vs. alcohol, diazepam, placebo, nothing, and with alcohol
- 70 professional drivers from the Finnish Army. Drove for 40 minutes to get rid of temporary alertness effect.
- Drugs given in a double-blind manner.
- 10 mg of diazepam, 50 mg of codeine, or placebo
- Results
- Zeros
- Generally felt their performances were normal. These results were used for the comparisons.
- Placebos
- 60% felt their performance was impaired and that their treatment was a tranquilizer and alcohol.
- Placebo subjects assessed their speed less accurately.
- Steering wheel reversals were significantly higher.
- Switched on turning signals much later.
- 1 collision in zero group vs. 3 in placebo
- Alcohol
- 50% felt their performance was impaired
- 90% recognized alcohol and 60% thought they also had a tranquilizer.
- Drove faster and slightly overestimated their speed.
- Steering wheel reversals were more common and the brake was used more often.
- Pulse reaction smaller during emergencies.
- Neglected instructions were more common and there more collisions.
- 50% felt their performance was impaired
- Diazepam
- Subjective performance was slightly impaired.
- 70% of subjects thought they received a tranquilizer.
- 80% thought they received alcohol.
- Generally drove faster and they tended to overestimate speed.
- Pulse reaction was smaller during emergencies.
- Codeine
- Subjective performance was slightly impaired.
- Considered both a tranquilizer and a stimulant of 40% of subjects.
- 60% of subjects thought they had also received alcohol.
- Average speed didn’t differ from zero group, but subjects slightly overestimated their speed.
- Number of steering wheel reversals was less.
- Pulse reaction was smaller.
- Significantly more collisions.
- Alcohol added to the other drugs
- Alcohol with diazepam
- Lowest speed and overestimated their speed the most.
- Number of steering wheel reversals was greater.
- Neglected instructions and caused collisions more often.
- Also drove off the road.
- Alcohol with codeine
- Generally felt performance as impaired, 80% of them thought they got a tranquilizer, and 90% of subjects recognized alcohol.
- Drove about as far as zero subjects, but slightly overestimated their speed.
- Commonly neglected instructions and drove off the road, and caused more collisions.
- Alcohol with diazepam
- Zeros
- Interpretation
- Alcohol, diazepam, and codeine led to increased risks during driving in emergency and monotonous surroundings.
(Bachs, 2009) – Evaluating the connection between vehicle accidents and codeine or tramadol
- Data from Norway
- 33-month study period yielded 181 accidents resulting in injury in drivers with codeine exposure (defined as within 7 days of the dispensation)
- And 20 drivers exposed to tramadol.
- Risk of accident was significantly higher for codeine drivers. The risk wasn’t significantly higher for tramadol drivers, but there was an upward trend.
- When removing members of the codeine group who were simultaneously exposed to other impairing drugs (other opioids, benzodiazepines, hypnotics, carisoprodol, and GHB), there was no longer an increased risk.
- Low vs. high consumption
- Low (filled prescriptions for fewer than 60 defined daily doses) vs. high (filed prescriptions for 60+ defined daily doses) in the 6-month period prior to the observation period.
- Risk of accident was significantly higher for the high-consuming group, but not for the low-consuming group.
- However, the higher risk among high-consumers went away when excluding those also exposed to other impairing drugs.
- Of the 83 high-consumers, 65 were also on other impairing drugs.
Nonmedical
Like other opioids, it can provide a state of relaxation, reduced care for physical or emotional pain/problems, euphoria, and even a dream-like state that enables easy shifting into semi-awake dreams.
Ceiling dose
It needs to be investigated further, but there seems to be a ceiling dose beyond which the recreational effects and analgesia don’t increase, while the negative effects could increase. This may stem from a CYP2D6-related mechanism or from a high occupancy of MOR by codeine, not by the more important metabolites.
This dose is somewhere around 300 – 500 mg for most people.
(Houde, 1965) – Suggested over 240 mg of codeine (IM) may have a decreased slope for analgesia.
Ultrarapid metabolizers could bypass this ceiling effect.
Chemistry & Pharmacology
Chemistry
Codeine is a naturally occurring methylated morphine derivative, specifically 3-methylmorphine. It’s an alkaloid and a member of the morphinan class.
The drug is found in opium at a concentration of 0.2 – 0.8%.
Though it can be collected from opium, it’s now synthesized from morphine.
Pharmacology
Before metabolism, it’s effectively inactive, with just minimal MOR affinity. The drug is best viewed as a prodrug, with metabolism to morphine and codeine-6-glucuronide (C6G) controlling its efficacy.
MOR is found along ascending and descending pain transmission pathways. Agonsim around the brain is associated with analgesia, respiratory depression, euphoria, miosis, and sedation.
Agonism of MOR in the midbrain is thought to be a core mechanism of opioid-induced analgesia. MOR agonists will indirectly stimulate descending inhibitory pathways acting upon the PAG and nucleus reticularis paragigantocellularis (NRPG), with the net effect of activating of descending inhibitory neurons.
This leads to greater neural traffic through the nucleus raphe magnus (NRM) and stimulation of 5-HT and enkephalin-containing neurons that connect directly with the substantia gelatinosa. In turn, this reduces nociceptive transmission from the periphery to the thalamus.
Opioids can also exert direct inhibitory effects on the substantia gelatinosa and peripheral nociceptive afferent neurons, reducing nociceptive transmission from the periphery.
Affinity
- Codeine (in guinea pig)
- 2,700 nM at MOR, over 10,000 at DOR, and undetermined at KOR
- Codeine (in rat brain tissue) (Srinivasan, 1997)
- 1,500 nM at MOR
- Codeine-6-glucuronide (in rat brain tissue) (Srinivasan, 1997)
- 4,400 nM at MOR
- Morphine (in guinea pig)
- 1.8 nM at MOR, 90 at DOR, and 317 at KOR
Importance of MOR
MOR-knockout mice show a lack of analgesia, respiratory depression, naloxone-precipitated withdrawal, and reward from morphine. Morphine actually becomes aversive, possibly from weak KOR activity.
Pharmacokinetics
Tmax: 60 minutes
Half-life: 2 – 3 hours
Codeine undergoes hepatic glucuronidation and N-demethylation. A small portion is converted to morphine and then to another active metabolite, morphine-6-glucuronide, which is more potent than morphine. Significantly more morphine goes to morphine-3-glucuronide (about 5x more), which is essentially inactive. Therefore, not enough morphine-6-glucuronide is produced to really have an effect after codeine administration.
Typically just 5-10% of codeine becomes morphine. A 30 mg dose is considered equal to 3 mg of morphine.
The greatest metabolite is codeine-6-glucuronide (C6G), which arises from the action of the UGT enzyme. It’s been suggested by some that C6G is the primary active metabolite since it reaches a higher concentration. However, a lot of evidence suggests morphine is the most important metabolite and it does indeed have a much higher MOR affinity. Codeine may also metabolize to morphine centrally, meaning the peripheral concentrations (from which we get 5-10% figure) could be underestimating the total exposure to morphine. Codeine may cross the BBB easier than morphine (Oldendorf, 1972).
Research does indicate C6G has its own analgesic effects.
Importance of morphine
Codeine’s analgesia is minimal in CYP2D6 poor metabolizers (PMs), but adverse effects (sedation, nausea, and itching) persist (Eckhardt, 1998). GI motility is also seemingly affected just in extensive metabolizers (EMs) (Mikus, 1997).
(Mikus, 1997) – GI motility fails to chance in CYP2D6 PMs
- Opioids typically delay gastric emptying and cause spastic constipation mediated through a direction in the GI motility and an increase in muscular tone in the GI tract. This comes directly via gut opioid sites and from some MOR sites in the brain and spinal cord.
- In EMs, the orocecal transit time was significantly prolonged by codeine. No significant difference was seen in PMs.
- PK
- Codeine serum concentration profile was nearly identical in EMs and PMs.
- While the morphine serum profile was significantly different.
- Peak morphine dropped from 13.9 pmol/mL in EMs to just 0.68 pmol/mL in PMs. And the AUC was just 7% of that in EMs.
(Fagerlund, 2001) – Case with minimal response due to PM status
- 65-year-old female given paracetamol and codeine for pain
- Minor analgesic effect and increasing the dose led to no extra analgesia, just extra side effects.
- Genetic testing showed complete lack of CYP2D6
CYP2D6 polymorphisms
CYP2D6 is highly polymorphic across populations, leading to both PMs and ultrarapid metabolizers (UMs). UMs could more easily generate dangerous levels of morphine. Extensive (normal) metabolizers make up the majority of the population.
Ethnicities
- PMs are overrepresented in those with northern European Caucasian descent.
- This group initially received the most attention due to the lack of efficacy in them.
- 7-10% of Caucasians, 2% of Asians, and 1% of Arabs.
- Ultrarapid metabolizers have 2 or more CYP2D6 gene copies, leading to enzyme activity score of 3 or higher.
- Can, at medical doses, sometimes result in respiratory depression or apnea.
- Frequency:
- 29% of those with African/Ethiopian heritage
- 21% for Saudia Arabia and other Middle East heritage
- 3.4% to 6.5% of African-American and Caucasian
- Also, CYP2D6 enzyme activity is low in newborns, developing to reach 60% of an adult by 28 days.
PK combination interactions
- CYP2D6 inhibitors (reducing efficacy)
- SSRIs (fluoxetine, paroxetine), duloxetine
- CYP2D6 inducers (increasing efficacy)
- Rifampicin, dexamethasone
Renal insufficiency
- The dose should be lowered.
Pharmacokinetic studies
(Poulsen, 1996) – Comparing the PK and analgesia of codeine and morphine in CYP2D6 PMs and EMs
- 14 EMs and 14 PMs
- Results
- PK
- In PMs, neither morphine nor morphine-6-glucuronide could be detected in plasma, except at very low levels in one measurement from one subject.
- Whereas for EMs, morphine was found in all subjects except for one, and morphine-6-glucuronide was found in all subjects in at least one measurement.
- Pain test
- For EMs, peak pain during the cold pressor test was significantly reduced by both codeine and morphine.
- For PMs, peak pain declined with morphine, but there was no effect of codeine.
- PK
- Interpretation
- PMs don’t produce any notable levels of morphine and they also fail to show an analgesic effect of codeine in the cold pressor test, which was shown to be sensitive for opioid analgesia with morphine in both groups. This suggests morphine is important for the analgesic effect.
(Srinivasan, 1997) – Exploring the analgesic effect of codeine-6-glucuronide
- Background
- Preview work has shown ICV administration of codeine and C6G produce significant analgesic effects in rats. C6G exhibited marked analgesic effects in these tests, up to 89% of morphine. The effect was greater than that seen with codeine, which led to 63%.
- This study looked at the affinities, absorption of C6G across BBB, and analgesia after IV administration
- Results
- IV administration of each drug led to a profound analgesic response
- After IV administration of codeine, morphine-3-glucuronide and morphine-6-glucuronide, plus unchanged codeine, were found in the plasma.
- In the brain, small levels of morphine-6-glucuronide and C6G were detected.
- Analgesia – Maximum possible effect after IV administration and peak response time (tail flick response time test)
- Codeine
- MPE of 98% and peak time of 20 min
- C6G
- MPE of 55% and peak time of 30 min
- Codeine
(Lotsch, 2006) – Evidence for CNS effects of codeine independent of morphine formation
- Background
- Inhibition of CYP2D6 by quinidine didn’t always remove the analgesic effects of 100 mg codeine, despite a very low morphine concentration.
- Subjects with non-functional CYP2D6 had side effects from 170 mg codeine.
- In 4 patients without CYP2D6 activity, an analgesic effect of 90 mg codeine was reported.
- Prolongation of GI transit from 50 mg codeine reported to not be dependent on morphine formation.
- Morphine-independent metabolites like C6G and norcodeine, along with codeine itself, are potential mediators of effects like cognitive impairment, sedation, dizziness, euphoria/dysphoria, headache, blurred vision, itching, flush, analgesia, and prolongation of GI transit.
- Study
- 16 healthy volunteers
- 2 had a CYP2D6 PM phenotype
- AUCs of the miotic effect were greater after codeine than after morphine. Yet, the plasma concentration for morphine after both were within the same general range.
- AUC of miosis was 1.7x greater for codeine than for morphine. This was despite similar or lower levels of morphine after codeine.
- The degree to which the AUC from codeine was more pronounced was greater in PMs compared to EMs, though not a significant difference between the groups.
- Attempting to fit pupil size changes to agonism of morphine + a second opioid
- This led to a highly significant improvement of fit. Every additional opioid in the analysis led to an improvement of fit vs. morphine alone.
- Greatest improvement from C6G, followed by codeine and norcodeine, then smallest effect from normorphine and M6G.
- 16 healthy volunteers
- It appears C6G and codeine, or potentially just codeine, could be responsible for some of the effects of codeine that aren’t attributable to morphine alone.
(Quiding, 1986) – PK of codeine and morphine after single and repeat codeine administration
- 12 male volunteers
- Each received two 60 mg doses of codeine 2.8 h apart.
- There forward, codeine 60 mg was taken regularly every 8 hours for a further 5 doses.
- Results
- Plasma concentration of codeine was about 40x higher than morphine. 1 subject showed no detectable morphine at any point.
- Compared to the first dose, the plasma concentration was significantly increased after the 2nd and 7th doses.
- For morphine, there was no difference between the 1st and 2nd doses, but the maximum concentration was significantly higher after the 7th doses.
- PK of codeine
- Cmax
- Dose 1 Cmax: 115 ng/mL
- Dose 2 Cmax: 136 ng/mL
- Dose 7 Cmax: 149 ng/mL
- Dose 1 Tmax: 1.2 hours
- Cmax
- PK of morphine
- Cmax
- Dose 1 Cmax: 2.4 ng/mL
- Dose 2 Cmax: 2.7 ng/mL
- Dose 7 Cmax: 3.8 ng/mL
- Dose 1 Tmax: 1.1 hours
- Cmax
- Half-life
- Codeine
- 2.2 hours after the second and 2.5 hours after the 7th doses.
- Morphine
- 2.9 hours after the second and 4.2 hours after the 7th doses.
- Codeine
- Adverse
- 3 reported adverse effects in connection with the 7th dose.
- 1 had constipation, 1 had dizziness, and 1 had relaxation
- 3 reported adverse effects in connection with the 7th dose.
Poppy seeds
Detectable opioid levels can be obtained from the ingestion of poppy seed bagels, though the concentrations aren’t high enough to cause noticeable effects.
(Hayes, 1987) – Concentrations of morphine and codeine after ingestion of poppy seeds
- Black poppy seeds from retail stores in Portland, Oregon
- Seeds had 17-294 ug/g for morphine and 3-14 ug/g for codeine.
- Human trial involving 4 healthy adults given the seeds with juice or yogurt
- 25 gram oral dose of poppy seed was provided, yielding approximately 7.5 mg of morphine and 0.4 mg of codeine
- Serum concentration of morphine averaged 100 ug/L (82-131) 2 hours after the dose and 6 ug/L (3-10) after 24 hours.
- Serum concentration of codeine averaged 7 ug/L (4-11) after 3 hours.
(Struempler, 1987)
- Poppy seed bagels purchased from a local bakery. Seeds removed from three bagels and subjected to analysis. Then, a healthy adult ate three bagels to examine the PK data.
- 5 total grams of seeds were recovered.
- Results
- Seeds
- Morphine: 963.6 ng/g
- Codeine: 78.8 ng/g
- Therefore, someone having a single bagel could ingest up to 1.5 mg of morphine and 0.1 mg of codeine.
- Urine
- Measurable amounts of codeine and morphine for up to 22 h after intake
- Morphine: Ranged from 246 ng/mL to 2797 ng/mL during that period
- Codeine: Ranged from 14 ng/mL to 214 ng/mL
- Seeds
Endogenous
Evidence points to the existence of a mammalian biosynthetic pathway for morphine and codeine that’s similar to the one found in P. somniferum. Codeine and morphine appear to be endogenous drugs, albeit without a clear function. L-tyrosine may be the starting material and codeine acts as a precursor to morphine.
(Donnerer, 1986) – Endogenous codeine and morphine identified in rat brain.
- To support the role of a P. somniferum-like pathway, rats received IV salutaridine, thebaine, and codeine.
- These led to a marked rise in morphine and codeine in rat tissues. Indeed, they’re also intermediates in the biosynthesis of morphine in P. somniferum.
(Weitz, 1986)
- Three morphinans were consistently found in extracts of bovine hypothalamus and variably in extracts of bovine adrenal and rat brain.
- Two of those were determined to be morphine and codeine, confirming their endogenous presence.
- However, fundamental questions remain unanswered. Namely, are they arising from an exogenous source and do they play a role in brain function.
(Neri, 2004) – Endogenous morphine and codeine in nonhuman primate brain
- Male nonhuman primate Chlorocebus aethiops
- Results
- Brain content of morphine: 0.11 ng/g
- Brain content of codeine: 0.04 ng/g
- Basal value in vitro for release over 10 min was 82.5 pg/g/min
- During a 5-min exposure of monkey brain blocks to a high potassium depolarization medium, the morphine concentration went to 170 pg/g/min (215% higher than basal)
- Basal morphine concentrations were restored when the high potassium medium was replaced with a normal medium
- When calcium was omitted from the superfusion medium, the depolarizing concentration of KCL failed to increase the rate of endogenous morphine release.
- This supports the Ca-dependent manner of release.
- Interpretation
- In the monkey brain, morphine and codeine are detectable. Codeine could be a biosynthetic precursor in the brain.
- The function of endogenous morphine is unclear. It could have general immune, vascular, and nervous roles.
- Potassium depolarization can cause the release of morphine in a Ca-dependent manner, supporting its role as a neuromodulator or neurotransmitter in nonhuman primates.
History
The history of isolated codeine is a couple centuries long, but the drug technically has a much longer history due to its presence in P. somniferum, which also directly contains morphine.
3000 BC
P. somniferum may have been cultivated for opium by the Sumerians living in today’s Iraq. They called opium “gil,” meaning joy. The poppy was called “hul gil,” meaning plant of joy.
1500 BC
The Ebers Papyrus from Egypt included a “remedy to prevent the excessive crying of children.”
Spenn, the grains of the spenn (poppy)-plant, with excretions of flies found on the wall, strained to a pulp, passed through a sieve and administered on four successive days. The crying will stop at once.
This remedy and others were sometimes considered dangerous due to variable potencies and rates of absorption.
700s – 900s
Arab traders likely brought opium to India and China.
The Arabian system of medicine, including opium use, was more clearly introduced to India by Muslims around the 800s – 900s.
973
Opium was mentioned in the Chinese medical book “K’ai-pao-pen-tsao.”
900s – 1200s
Over a period of a few centuries, opium spread from Asia Minor to all parts of Europe.
1500s
Manuscripts describing opium abuse and tolerance to opium appeared in Turkey, Egypt, Germany, and England.
1832
Pierre Robiquet, a French chemist, isolated codeine from opium for the first time.
1899
Lochboehler noted in JAMA that no case of fatal poisoning had been recorded or at least none could be located.
1934
At this point, codeine was typically considered nonaddictive, but some discussions challenged that idea.
(Himmelsbach, 1934) – Looked at the physical dependence potential of the drug
- 7 males with morphine addiction were stabilized on four daily morphine injections over a 10-30 day period
- Over a 3-day period, they were switched to codeine.
- Stability on codeine was maintained for 8-14 days before abrupt withdrawal
- Results
- Each dose of codeine became 240 to 600 mg, switching from 50-150 mg of morphine per dose.
- During the transition between drugs, mild abstinence symptoms were seen by all.
- During the codeine stabilized period, all were thoroughly stable for the majority of the time
- Abrupt withdrawal of codeine
- Mild abstinence during the first 30 hours, followed by severe symptoms in all cases until the 4th-5th day, when recovery set in.
- Only difference from morphine was the delayed onset of severe symptoms.
- Ratio of daily effective substituted doses:
- Ranged from 3.8:1 to 8:1, with a median of 5:1
1937
Gonzales estimated the lethal dose as 4.5 to 8 grains (291 to 518 mg) though they also mentioned reports of recovery from larger amounts.
1943
Krueger mentioned a death associated with codeine in a patient with acute asthma. That case was reported by Cohen in 1932, but no dose was given.
1960s onward
It’s been used in a widespread manner for pain and for cough.
Frequently it’s viewed as a good oral analgesic in outpatient settings, despite the evidence not actually providing an adequate reason for its widespread use.
And in the case of cough, codeine has long been viewed as the “gold standard” cough suppressant. Yet, the evidence doesn’t support that perception. It’s no more effective than placebo in many cases.
1960s – 1970s
In the US, more attention was given to a growing trend of recreational cough syrup use among teenagers. They would use easily accessible codeine-containing products.
The Wall Street Journal in 1968 had a story called “Cough Syrup Kicks.”
(Winek, 1970) – Talked about the use of codeine cough syrups by high school and university students. The cough syrup was often called “Robe” and sometimes people accidentally used non-codeine cough syrups containing dextromethorphan.
1997
The American Academy of Pediatrics was already skeptical of its use for pediatric cough.
No well-controlled scientific studies were found that support the efficacy and safety of narcotics (including codeine) or dextromethorphan as antitussives in children.
1997 – Nonmedical use in India
(Mattoo, 1997) – Discussed the use of and addiction to codeine-containing cough syrups in India
- Background
- There was reporting on the “abuse” of codeine-containing syrups in the lay and medical press. It was discussed in India Today in 1993 and in The Tribune in 1995.
- Reports from users, pharmacists, social workers, etc indicated Phensedyl was especially popular among teens and students.
- There was reporting on the “abuse” of codeine-containing syrups in the lay and medical press. It was discussed in India Today in 1993 and in The Tribune in 1995.
- Usual formulation (5 mL): 9-15 mg codeine, 5-7.5 mg ephedrine, and either 1-4 mg chlorpheniramine or 3 mg promethazine
- Recommended therapeutic dose: 15 to 30 mL in divided doses
- At an addiction center in North India, the Drug De-addiction and Treatment Center of the Department of Psychiatry at the Postgraduate Institute of Medical Education and Researcher (Chandigarh)
- 18-month period from January 1994 to June 1995
- 261 new substance abuse cases
- 126 were opioid abusers. Of these, 46 fulfilled the criteria for codeine-containing cough syrup dependence
- Mean age: 26.8
- CCS use started at a mean of 22.7 and had been going for a mean of 48 months
- Initiation started through friends (89%) or chemists/practitioners (11%)
- Reasons for initiation: Curiosity (63%), non-availability of other regularly used substance (22%), or treatment of a chest/cough ailment (15%)
- They progressed to daily use in under 1 month in 54% of cases, in 1-5 months in 35%, and over 6 months in 11%
- 46% took 5-6 doses daily, 36% used 2-4 doses, and 18% had a single daily dose
- Daily consumption ranged from 60 mL to 500 mL (50% of patients were using 200 to 500 mL daily)
- 93% of participants obtained their suppliers without a subscription and at 1.5-2x the normal retail price.
- 261 new substance abuse cases
- Effects
- 96% felt alert, 94% became more active, 94% became more cheerful, 76% become over-talkative, and 50% felt drowsy later on.
- They tended to report a distinct effect from codeine due to the stimulant and antihistamine combination. One patient said “pure codeine makes one dull, calm and placid; if one adds Ephedrex (a cough remedy containing ephedrine) to it, one becomes active, alert and experiences a prolonged kick.”
- Withdrawal
- 92% reported a withdrawal syndrome, typically with an onset of 4-48 hours (within 4-14 hours in 70%)
- Duration of symptoms was 1-5 days (4-5 days for 75%)
- 100% insomnia; 98% had body aches, lacrimation, and rhinorrhea; 83% had anxiety; and 78% had loose motions
- Psychiatric comorbidity
- 72% reported concurrent use of other substances
- 42% benzodiazepines, 12% other opioids, 10% alcohol, and combinations of these and/or carisoprodol in 36%
- 72% reported concurrent use of other substances
1990s to Current – The rise of codeine/promethazine or codeine-containing cough syrup use in the context of “purple drank” and “lean”
“Lean” and “purple drank” refer to a combination of codeine, promethazine (an antihistamine), soda/juice, and candy. Sometimes the soda/juice and candy are left out.
It first became a drug of choice among young African-Americans in the southern US, spreading outward from the Houston, Texas region in the early 1990s. It has since become a known drug around the US.
During its history, it’s been highly associated with the hip-hop music scene. Musicians like Lil Wayne have brought attention to its effects, often glamorizing the substance.
There’s a tendency to underestimate its harm potential.
At least two members of the Texas hip-hop community, Robert Davis Jr (DJ Screw) and Chad Butler (Pimp C), died of causes partly attributable to purple drank. Davis died in 2000 and Butler died in 2007.
Some high-profile arrests and media coverage of the substance increased public knowledge about the nonmedical use of codeine.
Johnny Jolly, a former Green Bay Packer, was arrested in 2008 for possessing 200 grams of codeine. In 2010, former Oakland Raiders quarterback JaMarcus Russell was arrested for possession of codeine. The next year, ESPN ran a special on purple drank and codeine that highlighted its apparent popularity among athletes.
Papers
(Peters, 2003) – Up to 25% of at-risk youth in Houston, Texas have used codeine cough syrups, with 10% reporting use in the past 30 days.
(Peters, 2003)
- Large and diverse alternative high school in the southwest US.
- 587 high school and middle school students invited.
- Of the 87 who returned completed parental consent forms, 56 reported being current cough syrup users.
- Of the 56, 48 were codeine-promethazine users and therefore eligible for the study
- 81% male
- Why do you believe codeine promethazine is so popular
- Both genders considered “media modeling” the top reason
- Also euphoric effects, peer pressure, and accessibility.
- Why do people use it
- Both reported euphoric effects and self-medication/coping
- Boys also reported peer pressure, cool/image, and cocktail drug
- Where do people get it from
- Both said doctors were the primary route.
- Females also said “unethical doctors” and “unethical parents/child prescriptions”
- Males also said “drug dealers/prescription acquisition” “unethical doctors” “friends” and “Stealing”
- What do people drink it with
- Typically mixed with soda or candy.
- What do your friends feel about people who use codeine promethazine
- Most said their friends considered it “normal” and males also noted “cool/image”
- Why do you believe you drank codeine promethazine the first time
- Both said peer pressure, curiosity, and euphoric effect.
- Males said curiosity was the top reason. For females, it was peer pressure.
- How long was it before the second time you drank codeine promethazine
- Less than one day for most.
- How many times does it take for someone to be addicted to codeine promethazine
- Most said first time or second time.
- When do you know someone is addicted to codeine promethazine
- Insomnia was the top response from both.
- For females, also “recurrence” and “stealing”
- For males, “aggressive behavior,” “withdrawal/craving,” and “recurrence.”
- What is a barrier to people quitting codeine promethazine use
- Both said “accessibility” and “peer pressure.”
(Peters, 2007) – Connection to sexual activity
- Cross-sectional study of male high school students in Houston, Texas.
- Current sexual activity was significantly associated with codeine cough syrup use, even after controlling for other drugs and other variables associated with past-month sexual activity.
- Results aren’t considered generalizable beyond the Houston youth population.
(Peters, 2007)
- Large historically Black university in the southwest US.
- Total of 307 students invited.
- 61 were current users.
- 56% male and 70% black.
- 61 were current users.
- What influenced you to use syrup?
- Peer pressure: 71% males and 72% females
- Curiosity: 14% males and 16% females
- Why do people use it
- Euphoric effect: 58% males and 72% females
- Self-medication/coping: 25% males and 5% females
- Where do people get it
- Doctors: 36% males and 29% females
- Pharmacist: 26% females (second top source)
- Friends: 26% males (second top source)
- What do people drink it with
- Most use it with Sprite or other soda or fruit juice.
- 8% of males and 12% of females took it with alcohol.
- No males took it straight, but 12% of females did.
- What do your friends feel about people who use codeine promethazine
- Cool image: 61% of females
- Felt it was a normal thing: 63% of males
- What other drugs might you use with codeine promethazine
- Cannabis: 70% males and 75% females
- Xanax: 19% males and 15% females
- Ecstasy: 8% males and 10% females
- What are some consequences of codeine promethazine
- Drowsiness: 55% males and 54% females
- Damage to organs: 27% males and 31% females
- Death: 10% males and 4% females
- Addiction: 7% males and 9% females
- What is a barrier to people quitting codeine promethazine use
- Males
- Withdrawal (45%), addiction (32%), and peer pressure (22%)
- Females
- Addiction (69%)
- Males
(Agnich, 2013) – Characteristics of codeine cough syrup (purple drank) users
- Large public university in the southeastern US. Fall 2011 and Spring 2012.
- Large, residential campus in a rural town. First survey outside of the Houston-area student population.
- Around 25% of students at the university come from the metro Atlanta area.
- 2,349 students completed the survey, leading to a response rate of 80.4%.
- Results
- 152 students (6.5%) used the drug.
- Use was significantly more common among males. The proportion that had used at least once was 9.3% among males vs. 3.9% for females.
- Asian Americans (5.1%), African Americans (5.4%), and Whites (6.1%) self-reported the lowest use.
- Hispanics (15.6%) and Native Americans (16.7%) reported the highest use.
- People raised in major urban environments, such as Atlanta (GA), had significantly greater use (12.2%) than those raised in suburban areas or moderate sized cities (7.3%). Both had higher use than people from rural areas (4.0%).
- Most other characteristics (age, class year, Greek status, family income, employment status, marital status, and athletic status) had no connection to purple drank use.
- Trend with use for GPA
- 5.1% in high GPA, 8.4% in second highest GPA, and then 9.9% and 28.6% in the lower two categories.
- Other drug use
- All users had taken alcohol in the past month.
- Cannabis users had a 10.7% rate vs. cannabis non-users at 0.7%
- Overall, students with lower GPAs, LGBT status, and cannabis/alcohol use were more likely to take purple drank.
- When controlling for substance use in the past month, gender and being raised in an urban environment were the only significant predictors.
2003
(Myers, 2003) – Study of 9,063 patients from 23 specialist substance abuse treatment centers in Cape Town, South Africa
- 1.25% of admissions were for people using analgesics as a secondary substance of abuse. Among that group, 40.4% reported the misuse of codeine products.
2010s
There’s been a strong pushback against the use of codeine in children and even in adults. This is based on respiratory and other health concerns, questions about its efficacy, and a few deaths in medical settings.
Concerns also exist with other opioids, yet there’s a good argument in favor of making the transition from codeine to morphine, oxycodone, and others. Some hospitals, such as Boston Children’s Hospital, have removed codeine from their formulary.
Many papers have been published opposing the continued widespread use of codeine.
Changes in the area of analgesia clearly need to be made regardless of whether people think codeine is a useful drug. (Taylor, 2008) found 64% of children at Toronto’s Hospital for Sick Children had moderate/severe pain in the 24 h before the interview, with 23% in significant pain at the time of the interview. This suggests pain management needs significant improvement.
National and international organizations have called for greater limitations on the use of codeine.
- March 2011 – WHO removed codeine from its list of essential medications for children due to concerns that its “efficacy and safety were questionable in an unpredictable portion of the pediatric population.”
- August 2012 – FDA issued a safety alert regarding the use of codeine in children after tonsillectomy, adenoidectomy, and adenotonsillectomy.
- February 2013 – Update from FDA added a “black box warning” to codeine and codeine-containing preparations. Warning advises prescribers “to prescribe an alternative analgesic [to codeine] for postoperative pain control in children undergoing tonsillectomy and/or adenoidectomy.”
- June 2013 – European Medicines Agency recommended restricting codeine for analgesia to children over 12-years-old and contraindicated its use in children under 18 undergoing tonsillectomy and/or adenoidectomy.
- June 2013 – Health Canada announced a review of the safety of analgesics and antitussives recommended against codeine in those under 12-years-old.
- March 2015 – European Medicines Agency review recommended against codeine in those under 12-years-old as well as in those from 12 to 18 who have breathing trouble.
2000s to 2010s
Codeine prescribing didn’t decline to any notable degree during the entire 2000s, despite growing calls for a shift.
In the pediatric ED, codeine was given at a rate of 558,805 to 876,729 prescriptions per year from 2001 to 2010, according to data from Kaiser’s “National Patterns of Codeine Prescriptions for Children in the Emergency Department.” There’s only been a small statistical decline during that period.
Even though we’ve long known about a lack of proven efficacy as an antitussive for children, Kaiser didn’t find a change in the frequency of codeine prescribing for pediatric cough or cold. It ranged from 69,057 to 145,857 prescriptions annually.
In 2011, the FDA reported more than 1.7 million children 17-and-younger had a prescription for codeine filled at a pharmacy.
A 2011 study in the US found codeine was given to over 800,000 patients under 11-years-old, making it the most popular opioid in that demographic.
From 2007 to 2011, otolaryngologists were the most frequent prescribers of codeine/paracetamol liquid formulations (19.6%). They were followed by dentists (13.3%), pediatricians (12.7%), and general physicians (10.1%).
US poison control centers reported over 900 poisonings among children under 12-years-old in 2011. An additional 1400 were reported with analgesics or cough/cold preparations containing the substance.
2011 – The Patient Safety and Quality Improvement Committee of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) determined the availability of other analgesics and the risk of severe events from codeine outweighed the utility of codeine for adenotonsillectomy patients.
In 2013, based on Medical Expenditure Panel Surveys in the US, codeine was the top opioid in children, with over 1 million receiving a prescription. ED physicians and dentists were the most frequent prescribers.
2012
There were 3.6 million prescriptions in primary care for codeine in England. Most of the prescriptions were for 30 mg and 15 mg tablets. Far more prescriptions were reported for combination products.
2013
(Gill, 2015) – Survey of doctors in the UK about the switch from codeine to morphine.
- The MHRA published its restrictions on the use of codeine in children in July 2013.
- Many pediatric centers then switched to morphine from codeine. In the case of the evaluated center, that switch was in October 2013.
- Doctors were surveyed about their response to the shift.
- 280 contacted, but only 29 responded.
- Results
- 83% were happy with the info they received from the NHS and 88% were positive or neutral about the coordination of the switch.
- Of the 64 comments made about using morphine in place of codeine
- 77% were negative, 20% positive, and 3% neutral
- Most common concerns: increased risk of errors, abuse/ingestion potential in the community, lack of an intermediate analgesic, and reduced availability due to controlled drug restrictions.
- Positive responses focused on improved pain management, simpler regimes, and the variety of preparations giving greater flexibility.
2010s
(Fredheim, 2009) – Prescriptions for codeine in the context of analgesia increased in Europe.
(McAvoy, 2011) – Misuse of nonprescription codeine-containing medicines increased in Europe.
2010s
(Van Hout, 2015) – Review of the recreational use of codeine in Ireland
- Based on data from the National Drug Treatment Reporting System, codeine was the primary or secondary drug of abuse from 2008 to 2012 among 1.9% of persons in drug treatment in Ireland.
- (Cohen, 2009) – Irish misusers of codeine are more likely to be male, older, with comorbid psychiatric, physical, and polysubstance illness and with a longer dependence history.
- This paper involved interviews with 21 codeine misusers
- Mean age: 39
- 15/21 reported using codeine within the last 12 months and the majority scored 10 or above in the SDS.
- Many combined codeine with alcohol, especially at night time.
- Displacement to “stronger” opioids (Oxycontin and heroin) was reported by 2 participants.
- Majority reported preference for Nurofen Plus, but sometimes they’d use other OTC and prescribed options like Solpadeine, Feminex, Solpadol, Tylex, and Codinex.
- Awareness of habit forming use and harm
- Majority weren’t aware of the addictive potential of codeine-containing medicines and the harms of additives like ibuprofen and paracetamol.
- Just 2 reported reading the product info leaflet.
- Negotiating pharmacy sales
- All used pharmacies as their primary source. All described using multiple pharmacies in different locations and at various intervals in order to avoid suspicion.
- Alternative sourcing routes
- Other sourcing methods centered on diversion via prescriber, street, and family routes.
- Some people traveled to areas with less stringent regulations. 2 individuals used this method, going to Spain and Northern Ireland.
- 1 person described consulting multiple doctors and forging of scripts.
- The codeine feeling
- Initial use began due to physical pain like migraine, dental, back, menstrual, joint, postoperative, and child birth.
- Switching to nonmedical use tended to occur due to the pleasurable effects and using it for emotional distress and as a coping mechanism.
- One user – “Nothing stressed me when it worked, codeine filled a void.”
- Acute and chronic side effects
- Acute tended to involve urticarial itching, distorted vision, and respiratory depresion.
- Chronic centered around nausea, constipation, liver/bowel/kidney failure, anemia, seizures, ulcers, and swollen stomach.
2014
South African Community Epidemiology Network on Drug Use (SACENDU) data.
July to December 2014; collected at specialist drug treatment centers.
263 of 10,197 treatment admissions involved codeine as a primary or secondary substance of abuse. Of those, only 78 had codeine as their primary substance of abuse.
2015
(Van Hout, 2015) – Misuse of and dependence on codeine products in South Africa
- Patients recruited from clinics around South Africa.
- Total of 26 participants
- Mean age: 43
- 20/26 admitted misusing codeine in the last 12 months; majority scored 10+ on the SDS.
- 32% reported codeine tablets as their primary drug of use, 20% used codeine syrup, and 12% used both.
- Motives for codeine use
- Many began using it for pain, such as for arthritis, migraine, and severe headache, or postoperative.
- Many people used it to cover up depression, anxiety, and stress-related conditions.
- Transitioning from codeine use to misuse and dependence
- Initially, they tended to think their use was appropriate and legitimate. They were largely unaware of codeine’s addictive potential or of the harms associated with additives.
- Daily use progressed within several weeks. Many people reported life stressors causing their use to quickly increase.
- Process of pharmacy purchasing
- Many didn’t really consider their substances to be “drugs” since they were freely available.
- Pharmacies were the primary source. All people decreased using multiple pharmacies in different locations and at various times to avoid suspicion.
- Diverse alternative sourcing routes
- Other methods involved internet purchasing and diversion via prescriber, street, and family. Sometimes travel to other less-restrictive jurisdictions.
- The Codeine effect
- Reported a “buzz,” and removal of stress, anxiety, or depression.
- Euphoria, relaxation, and feeling of fulfillment.
- Withdrawal experiences
- Fear, crying, self-pity, anxiety, irritability, aggression
- Insomnia, perspiration, shaking.
Legal Status
US
Codeine is Schedule 2. There are some formulations in Schedule 3 and 5.
Some polysubstance products are even sold OTC.
International
Typically it’s a scheduled drug. Yet unlike most scheduled drugs, some preparations with lower concentrations of codeine and other substances are sold OTC in some regions.
Australia
Schedule 2 and therefore available OTC: Product with under 10 mg codeine per dose.
Schedule 3 and therefore available behind-the-counter: 10 to 15 mg codeine per unit.
Schedule 4 and therefore prescription-only: 16 mg and higher per unit
Schedule 6 – Pure codeine products
The country will, on February 1, 2018, get rid of non-prescription codeine products. All will be Schedule 4 or Schedule 8.
Canada
Schedule 1
Though products with low concentrations are available OTC. This includes Tylenol 1, which has 8 mg codeine + caffeine and paracetamol.
UK
Class B. It’s illegal without a prescription.
Combos with small concentrations under 12.8 mg are available OTC.
Safety
Overdose
An overdose can come with respiratory depression, unconsciousness, coma, and vomit aspiration.
Compared to other opioids, it may be somewhat more likely to produce seizures, delirium, mania, and other psychological changes. Though the seizure activity may just be coming from hypoxia.
Death, though relatively rare, occurs via respiratory depression. Ultrarapid metabolizers are at a higher risk of fatal consequences.
Problems at medical or near-medical doses in children
On rare occasions, medical doses given directly to young children or indirectly (i.e. through breastfeeding) have caused severe respiratory depression or death. These cases sometimes, though not always, involve ultrarapid metabolizers.
Children who require adenotonsillectomy often also have obstructive sleep apnea that’s not fixed by the surgery. As such, codeine use is contraindicated in that population. The FDA issued a warning about its use in that population in 2012.
FDA Adverse Event Reporting System from 1969 to May 2012
- 10 cases of pediatric death and 3 cases of respiratory depression associated with the therapeutic use of codeine.
- Age range of 21 months to 9 years
- Majority in postoperative period after adenoidectomy or adenotonsillectomy.
- Using recommended codeine dose and dosing interval.
(Friedrichsdorf, 2013) – 3 additional pediatric deaths
- 10-year-old who underwent orthopedic surgery
- 4-year-old after tonsillectomy
- Third child who received codeine as an antitussive, though at a supratherapeutic dose.
FDA Adverse Event Reporting System from 1965 to 2015 (specifically children using codeine or codeine-containing products)
- 64 cases of severe respiratory depression
- 24 codeine-related deaths
- 21 involving children under 12-years-old.
(Subramanyam, 2012) – Review of 233 pediatric malpractice claims from 1984 to 2010 after pediatric tonsillectomies
- 18% of deaths and 8% of nonfatal cases of opioid toxicity could be attributed to opioid use, most often codeine.
(Friedrichsdorf, 2013) – Fatal cases involving medical doses
- In all cases, examination of the medication bottles confirmed the intended dosing was given. Even in Case 2, where there was an error with the labeling, a medical dose was still dispensed.
- The measured blood concentrations exceeded the calculated estimate in every case.
- Case 1
- 10-year-old female
- With reactive airway disease and a history of snoring (indicative of obstructive sleep apnea)
- Underwent orthopedic surgery
- Given 20-40 mg of codeine every 4 hours as needed + diazepam 2-4 mg every 4 hours as needed for spasms + one dose of codeine 12 mg/paracetamol 120 mg in the afternoon, plus a second with diazepam at bedtime
- She was tolerant to benzodiazepines
- Mother set an alarm for 01:30 to give medications
- When she arrived, her child was cold and unresponsive. Emergency responders failed to resuscitate.
- Toxicology postmortem (concentrations were within the toxic range)
- Codeine total (heart blood): 0.78 mg/L
- Morphine (heart blood): 0.15 mg/L
- Determined to be a fatality from combined sedation from opioid and benzodiazepine doses. Plus obstructive sleep apnea and reactive airway disease as possible contributing factors.
- Case 2
- 6-year-old female
- History of myocarditis + new onset of severe cough and respiratory infection
- Given oral azithromycin and scheduled guaifenesin/codeine (100 mg/10 mg) leading to 10-20 mg of codeine every 4 hours.
- There was a prescription labeling area. She was supposed to just receive 10-20 mg at bedtime, but the label said every 4 hours.
- Received a dose at 07:00, 15:00, and 19:00
- Mother checked on the child at 19:45, noting she was “a little blue.”
- ED informed her that she should bring her daughter to the ED. Yet the mother didn’t.
- Next morning at 08:00, the mother entered her daughter’s bedroom and found her daughter lifeless, pale, and cold.
- Toxicology postmortem
- Codeine total (iliac blood): 0.17 mg/L
- Morphine (iliac blood): 0.08 mg/L
- Case 3
- 4-year-old girl
- Presented for tonsillectomy/adenoidectomy
- History of absence seizures, well-controlled with valproate
- Given codeine/paracetamol, leading to 12-17 mg codeine every 4 hours as needed for pain.
- 17 mg of codeine given at 08:00, 12:00, 16:00, and 20:00
- Found unresponsive the following morning.
- CYP2D6 testing confirmed she was an EM (normal)
- Toxicology postmortem
- Codeine total: 0.61 mg/L
- Morphine: 0.31 mg/L
(Ferreiros, 2009) – Fatal and severe codeine responses in young twins
- 3-year-old twin males
- Suffering from cold with fever for 6 days.
- One child was then found lying breathless in vomit at 02:40, but the other child showed no symptoms.
- Brought first child to hospital
- Was comatose and needed mechanical ventilation upon arrival.
- Ventilated for 3 days because of aspiration pneumonia, but recovered fully.
- Other child was then found dead by the father 2.5 hours after admission of his brother to the hospital.
- Lying in vomit and resuscitation was unsuccessful.
- Dose of codeine
- Given 10 drops of a slow-release codeine cough medicine for the past 6 days. Last administration was at 22:00 the same night.
- Toxicology
- Serum samples from the heart of the dead child (around 7 h postmortem) and periphery of the living child (around 6 h after the last intake)
- Deceased child
- Codeine serum: 436.3 ng/mL
- Morphine serum: 138.7 ng/mL
- Living child
- Codeine serum: 174 ng/mL
- Morphine serum: 25.6 ng/mL
- CYP2D6 testing indicated EM status
- Determination of the daily dose
- Mother said she gave 10 drops of codeine retard suspension daily rather than using the included spoon to measure the target dose of 0.5 mL (10 mg of codeine)
- When tested, this came out to an average of 21.2 mg and 16.8 mg of codeine, higher than the labeled amount.
- Explaining the discrepancy in the PK data between the children and how the dead child’s concentration was over 400 ng/mL
- Modified the PK model’s volume of distribution elimination half-life, and bioavailability due to the children being ill for 6 days (could impact metabolic capabilities) and due to them being quite thin (impacting volume of distribution.)
- Under these alterations, the maximum theoretical codeine concentration was 430 ng/mL
(Magnani, 1999) – Young patient
- 1-month-old received combination medications including codeine as an antitussive
- Developed severe respiratory depression.
- Total dose was 1.26 mg/kg during a 6 h period
(Koren, 2006) – Breastfeeding
- Breastfed baby died from respiratory depression
- Genotype analysis showed the mother had three functional CYP2D6 alleles, making her a UM.
- Analysis also showed a high morphine content in breast milk.
(Voronov, 2007) – Young patient
- 29-month-old, previously healthy
- Experienced apnea leading to brain injury following a dose of codeine given 2 days post-tonsillectomy
- Genetic polymorphism with UM status was proposed, but not confirmed.
Pregnancy
The use of codeine during pregnancy is estimated to be around 1% to 3.5%. It’s not currently contraindicated.
(Nezvalova-Kenriksen, 2011)
- Data from the Norwegian Mother and Child Cohort Study, released in 2008.
- 67,982 pregnant woman in the study
- 2,666 (3.9%) used codeine during their pregnancy.
- 45 took codeine alone, while 2,621 used codeine with paracetamol
- 2,666 (3.9%) used codeine during their pregnancy.
- Results
- No significant difference in survival rate.
- No significant difference in overall congenital malformation rate.
- No significant difference in major congenital malformation rate.
- Use at any time during pregnancy was significantly associated with an increase risk of acute Cesarean delivery and postpartum hemorrhage.
- The strongest association was between codeine use and a higher risk of planned Cesarean delivery. A dose-effect relationship was seen. However, this may not have been causal since the using group also had significantly higher rates of underlying medical conditions
Safety with combos
If not taken for medical reasons, it’s important to avoid combination products.
Combinations, such as with paracetamol or ibuprofen, come with a higher risk of GI hemorrhage, nephrotoxicity, hypokalemia, acute hemorrhagic necrotizing pancreatitis, and brain damage.
(Frei, 2010) – Problems associated with the misuse of OTC codeine-ibuprofen
- 27 patients in Australia
- Mainly had opioid dependence and GI complications (attributed to ibuprofen)
- 10 cases of each
- 3 had hypokalemia and 1 required dialysis. The complications (hypokalemia and renal failure) were tied to the use of high dose NSAIDs like ibuprofen.
- 26/27 reported prolonged (past 6 months) use of supratherapeutic OTC codeine-ibuprofen doses. Mean duration of 3.6 years.
- In the 15 cases where source was discussed, all patients used multiple pharmacies.
- Mean dose: 34 to 47 tablets daily, leading to 435 to 602 mg of codeine and 6800-9400 mg of ibuprofen.
- Nurofen Plus was the brand specified by 9 patients.
- 15 reported initiating their use due to painful conditions like back pain and headaches. They then escalated their dose.
Respiratory effects
Using too much, especially in people with a UM status, could result in fatal respiratory depression. It reliably alters respiration in a manner that can become easily dangerous when other depressants are co-administered.
(Bellville, 1967) – Comparing morphine and codeine
- 7 male healthy volunteers
- Results
- Didn’t use high enough doses to figure out if there’s a ceiling effect.
- A relative potency of about 10% of morphine for the same level of respiratory depression.
(Lasagna, 1954)
- Respiratory effects of codeine (60 and 120 mg) vs. morphine (10 mg) SC
- Same order of magnitude of effect
- Studying response to 5% CO2 following placebo, codeine 60 mg, codeine 120 mg, and morphine 10 mg
- While codeine 60 mg led to less respiratory depression than morphine 10 mg, codeine 120 mg produced more respiratory depression than morphine 10 mg.
Withdrawal
Physical dependence reliably develops with extended use. The severity of the withdrawal will depend on the dose and the duration of use.
Among the symptoms are insomnia, restlessness, runny nose, stomach pain, diarrhea, and chills. It can feel similar to a severe flu and sometimes worse.
Craving for the drug is also increased.
Hepatotoxicity
Though it’s not really a concern in clinical settings, in vitro tests suggest there’s some potential for hepatotoxicity. This isn’t typically a concern and we have little evidence of it happening to typical users.
(Ellington, 1987)
- Isolated rat hepatocytes
- Administration of codeine led to cytotoxicity shown by a dose- and time-dependent LDH leakage out of cells.
- Induction and inhibition of enzymes then used to figure out if a metabolite was responsible
- Toxicity wasn’t altered by phenobarbital’s induction of cytochrome P450
- Toxicity declined after cytochrome P448 induction by B-napthoflavone
- Toxicity inhibited by metyrapone, an inhibitor of cytochrome P450
- Inhibition of flavin adenine dinucleotide (FAD)-containing monooxygenase increase the toxicity.
- Results indicate codeine hepatotoxicity arises from a P450-generated metabolite, while FAD-containing monooxygenase may metabolize the drug to a nontoxic substance.
Acute psychiatric changes
There have been reports of opioids leading to depression, mania, and even psychotic states, especially in overdose. Some have historically been viewed as more likely to cause these effects. Codeine is considered more likely than some alternative opioids.
This is an issue some people appear more susceptible to.
(Kenneth, 1998)
- 52-year-old male
- 1 week prior – Experienced exacerbation of chronic lumbar pain and prescribed Panadeine forte (paracetamol 500 mg + codeine 30 mg)
- Ended up using more than prescribed. Estimated ingestion of 80 tablets over a 4 day period.
- Family described him as increasingly boisterous, buoyant, gesticulating, and speaking rapidly. His sleep deteriorated and the night preadmission he prayed for ~10 hours and claimed to have spoken to his dead mother.
- Referred to GP for mental assessment
- Overfamiliar; kissing the female accident and emergency registrar.
- Distractible, spoke past the point, and felt “fabulous.”
- Claimed he was one of the world’s top guitar players and that in his younger years “there was the Beatles, the Rolling Stones and us”
- Poor concentration; oriented to time, place, and person
- Only other medications: low-dose aspirin and salbutamol
- No psychiatric history, but a history of psychiatric responses to opioids
- 18 months prior – Postoperative phase of Nissen fundoplication and following the use of pethidine, he developed transient visual hallucinations and became “very moody,” weeping and laughing. Symptoms lasted under 48 hours.
- Similar episode several years prior – Described as “high,” singing happily for a day after receiving an IM analgesic (likely pethidine) following inguinal hernia repair.
- Also became “very mood and aggressive” after Panadeine forte for a dental abscess many years prior.
- Diagnosis
- Manic episode
- Treatment and outcome
- Thioridazine 100 mg at night with clonazepam for insomnia
- Sleep pattern returned to normal and over the following 5 days his distractibility, euphoria, overfamiliarity, pressured speech, and grandiose beliefs subsided.
- At 1-month followup: full recovery without medication.
(Malek-Ahmadi, 1985)
- 20-year-old male with no psychiatric history
- Became agitated and developed auditory hallucinations and paranoid delusions after 5400 mg paracetamol and 540 mg codeine over 36-hour period
- Symptoms resolved in under 72 hours
(Ishigooka, 1991)
- 44 individuals
- Mental disturbance from BRON (cough suppressant with methylephedrine, codeine, caffeine, and chlorpheniramine)
- Symptoms included “intermittent exhilaration similar to a panic state”
- Suggested codeine was the primary cause
Chronic use
One of the concerns associated with long-term use is depression, which could sometimes arise from androgen deficiency, a fairly common effect of chronic use. Androgen deficiency exists in up to 75% of males and females chronically receiving opioids.
Some brain changes in the nucleus accumbens and amygdala have been reported in certain papers.
(Scherrer, 2016) – Evaluating the new depression diagnosis risk
- 11,462 Veterans Health Administration patients prescribed only codeine, only hydrocodone, or only oxycodone for over 30 days.
- 22.9% given codeine, 63% given hydrocodone, 14.1% given oxycodone
- Results
- Patients prescribed only codeine for greater than 30 days had a 29% increased risk of a new depression diagnosis compared to those given hydrocodone.
- Those given oxycodone weren’t significantly more likely to get a depression diagnosis than those prescribed hydrocodone.
- Suggests patients only receiving codeine are at a higher depression risk.
Risky combinations
Other depressants, including benzodiazepines, other opioids, ethanol, pregabalin, GHB, and dissociatives.
Problems at medical doses
(Gasche, 2004)
- 62-year-old male
- History: chronic lymphocytic leukemia
- 3-day history of fatigue, dyspnea, fever, and cough
- Last received chemotherapy 3 months prior
- Only medication was valproic acid (1500 mg daily), which he had been on for years.
- Arrival: Alert and oriented and was hypoxemic, but normocapnic.
- Findings compatible with bilateral pneumonia limited to the inferior lobes.
- Because of his immunocompromised state, bronchoalbeolar lavage was performed.
- Preliminary results – No p. carinii, but yeast
- Therapy: Ceftriaxone, clarithromycin, and voriconazole. Plus oral codeine (25 mg three times daily) for cough.
- Hospital Day 4
- Deterioration of consciousness level, becoming unresponsive.
- Last dose of codeine 12 hours earlier.
- Acute respiratory distress syndrome with arterial blood gas of 56 mm Hg and FiO2 of 0.5
- Partial pressure of CO2 of 80 mm Hg
- Treated with noninvasive ventilation and transferred to ICU
- GCS of 6
- Miotic pupils
- 90 min after ventilation
- New arterial blood gas measurements showed partial pressure of CO2 of 56 mm Hg and partial pressure of oxygen of 68 mm Hg
- Though no neurologic improvement
- IV naloxone (0.4 mg twice) led to dramatic improvement in consciousness. Titration of naloxone (continuous perfusion of 0.4 mg per hour for 6 hours) led to a normal consciousness level and respiratory failure resolution.
- 2 days after acute event: Full recovery
- Toxicology
- At the time of coma
- Plasma codeine – 114 ug/L (max expected level of 13 to 75 ug/L)
- Plasma codeine glucuronide – 361 ug/L (expected range of 700 to 1670 ug/L)
- Plasma morphine – 80 ug/L (expected range of 1 to 4 ug/L)
- Plasma morphine-3-glucuronide – 580 ug/L (expected range of 8 to 70 ug/L)
- Plasma morphine-6-glucuronide – 136 ug/L (expected range of 1 to 13 ug/L)
- At the time of coma
- CYP2D6 genotyping showed UM status and this was confirmed with DXM metabolism profile.
- Other factors
- CYP3A4 mediates metabolism to inactive norcodeine. CYP3A4 was inhibited by the macrolide and azole derivatives, further reducing clearance and increasing risk of opioid overdose associated with CYP2D6 UM status.
- Norcodeine wasn’t even detected in the blood.
- CYP3A4 mediates metabolism to inactive norcodeine. CYP3A4 was inhibited by the macrolide and azole derivatives, further reducing clearance and increasing risk of opioid overdose associated with CYP2D6 UM status.
(Kuo, 2004) – Associated with seizures in an elderly female with renal insufficiency
- 73-year-old Taiwanese female with nephrotic syndrome and chronic renal insufficiency for 6 months
- She was on hemodialysis
- Eventually prescribed oral codeine 30 mg four times daily for back and rib pain following poor paracetamol response
- 7th day of receiving codeine
- Developed 3 tonic-clonic seizures at intervals of 3-4 hours, each lasting ~1 minute
- Experienced postictal symptoms including one episode of vomiting, drowsiness, and a severe headache.
- Treated with phenytoin 375 mg IV, followed by 100 mg maintenance dose 3 times daily
- Codeine continued
- 8th day
- One more seizure then sleeping for the entire day. Codeine was then discontinued.
- 9th day
- EEG showed diffuse moderate cortical dysfunction and no epileptiform discharge.
- Woman remained stuporous with pinpoint pupils.
- Given IV naloxone 0.4 mg, immediately leading to consciousness
- Continued with IV infusion of naloxone at 0.4 mg per hour for 10 hours, along with phenytoin 100 mg 3x daily
- No more seizures after codeine ended and naloxone began.
(Shipton, 1984)
- Seizures in a child believed to be from unintentional use of codeine-containing tablet.
(Talbott, 1997)
- 5-year-old male with chronic renal failure
- Respiratory arrest and tonic-clonic seizures after the use of paracetamol/codeine elixir
- Believed to potentially arise from morphine-6-glucuronide metabolite
(Zolezzi, 2001)
- 7-year-old male
- Tonic-clonic seizures after IV administration of codeine, which were then controlled with diazepam and naloxone.
- Seizures didn’t start until 7 days of codeine therapy, which had been oral
- No more seizures after codeine stopped and phenytoin and naloxone were given.
Fatalities
(Cornell, 1949)
- 30-year-old male with a history of heavy drinking and asthmatic symptoms
- Asthma attack in September 1947
- Showed “typical asthmatic breathing” and a “typical expiratory wheeze”
- Slight dyspnea
- Day before death (11th day in hospital)
- Consumed a pint of whiskey before 9 am
- Received phenobarbital at 9 am and 1 pm
- Afternoon – Seen drinking and later sat with other patients, at which point he was drowsy and fell asleep multiple times.
- 3.15 pm – Two “swigs” from a cough mixture bottole.
- 8:30 p.m. – Sleeping
- 1:10 am – Still sleeping
- 3 am – Snoring
- 5:50 am – Dead
- Autopsy performed 4.5 hours postmortem
- Moderate pulmonary congestion
- Investigation revealed the cough mixture accidentally had 7 grams of codeine, not 700 mg. Leading to 875 mg per ounce.
- Patient took an estimated 1 to 2 oz of the medicine, leading to 875 to 1,750 mg of codeine.
(Pearson, 1979)*
- 49-year-old female
- Week prior to death – Depressed and consuming a large amount of whiskey
- Day of death
- 4 am – Sleeping and snoring normally
- 8 am – Unable to be awoken by husband
- Dimetapp, aspirin, and Bufferin were reportedly the only medications in the house. Toxicology confirmed codeine was used.
- Toxicology
- 16 ug/mL
- The peak serum concentration after 15 mg codeine was reported to be 0.03 ug/mL by Schmerzler, meaning this case involved a concentration over 500x greater.
(Winek, 1970)
- 21-year-old male
- Arrived home around midnight and was acting oddly. Immediately went to bedroom and collapsed. Mother came in, found him frothing at the mouth and having trouble breathing.
- Taken to hospital by family, but pronounced dead on arrival.
- Autopsy
- Hemorrhagic bronchopneumonia, acute congestion and edema of lungs, acute passive congestion of spleen and liver, and cerebral edema.
- Toxicology
- Confirmed codeine, pheniramine, and alcohol.
- Sister indicated he was using a red cough syrup and empty Robitussin AC boxes were found in his room.
- Each four-once bottle of Robitussin AC has 240 mg of codeine. With ingestion of the three available bottles at once, he would have approached the alleged minimum lethal dose of 800 mg, though alcohol and pheniramine could have played a role.
Fatality series
(Dhalla, 2009)
- Opioid deaths in Ontario from 1991 to 2004
- Codeine was the only opioid used in 20% of patients.
- Some deaths may have come from intentional overdose and some may have come from genetic factors.
References
(2017) “Codeine Is My Helper”: Misuse of and Dependence on Codeine-Containing Medicines in South Africa.
(2017) Codeine: an old drug with new precautions.
(2016) New depression diagnosis following prescription of codeine, hydrocodone or oxycodone.
(2016) Codeine: Time To Say “No”
(2015) What do doctors working in pediatrics think about switching from codeine to morphine?
(2015) ‘Codeine is my companion’: misuse and dependence on codeine containing medicines in Ireland
(2015) Nod and wave: An Internet study of the codeine intoxication phenomenon
(2014) Why can’t we retire codeine?
(2014) Codeine, alone and with paracetamol (acetaminophen), for cancer pain
(2013) Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures.
(2013) New Evidence about an Old Drug — Risk with Codeine after Adenotonsillectomy
(2013) Is it farewell to codeine?
(2013) Codeine-associated pediatric deaths despite using recommended dosing guidelines: three case reports.
(2012) Basic opioid pharmacology: an update
(2012) Treatment of the Common Cold in Children and Adults
(2011) Effects of codeine on pregnancy outcome: results from a large population-based cohort study.
(2011) Recent updates on codeine
(2010) Has the time come to phase out codeine?
(2010) Codeine and cough: an ineffective gold standard
(2010) Single dose oral codeine, as a single agent, for acute postoperative pain in adults.
(2010) Pro–con debate: is codeine a drug that still has a useful role in pediatric practice?
(2009) Single dose oral paracetamol (acetaminophen) with codeine for postoperative pain in adults.
(2009) The risk of motor vehicle accidents involving drivers with prescriptions for codeine or tramadol.
(2005) Advances in opioid pharmacology.
(2004) Endogenous morphine and codeine in the brain of non human primate.
(2004) Probable codeine phosphate-induced seizures.
(2004) Codeine Intoxication Associated with Ultrarapid CYP2D6 Metabolism
(2003) Clinical pharmacology and rationale of analgesic combinations.
(2001) “Doctor shopping and pharmacy hopping”: practice innovations relating to codeine
(2001) No pain relief from codeine…? An introduction to pharmacogenomics.
(1998) Mania associated with codeine and paracetamol.
(1997) Effect of codeine on gastrointestinal motility in relation to CYP2D6 phenotype.
(1997) Paracetamol with and without codeine in acute pain: a quantitative systematic review.
(1997) Analgesic effects of codeine-6-glucuronide after intravenous administration.
(1997) Abuse of codeine-containing cough syrups: a report from India.
(1997) Assessment of the antitussive efficacy of codeine in cough associated with common cold.
(1996) Codeine, cough and upper respiratory infection.
(1993) A brief history of opiates, opioid peptides, and opioid receptors.
(1987) Excretion of codeine and morphine following ingestion of poppy seeds.
(1987) Concentrations of morphine and codeine in serum and urine after ingestion of poppy seeds.
(1986) Morphine and codeine from mammalian brain.
(1986) Treatment of narcolepsy with codeine.
(1982) Analgesic efficacy of an ibuprofen-codeine combination.
(1979) A fatality due to the ingestion of (methyl morphine) codeine.
(1974) Effects of diazepam and codeine, alone and in combination with alcohol, on simulated driving.
(1970) Codeine fatality from cough syrup.
(1968) The respiratory effects of codeine and morphine in man
(1951) Codeine poisoning: fatal case from accidental overdose.