 AH-7921 is a synthetic opioid with an atypical structure. Early studies indicated it had a similar potency to morphine, though reports in humans suggest the potency is lower, at least if you’re looking for recreational effects.
AH-7921 is a synthetic opioid with an atypical structure. Early studies indicated it had a similar potency to morphine, though reports in humans suggest the potency is lower, at least if you’re looking for recreational effects.
It has been detected in over a dozen deaths, though the majority of those cases involved other drugs, often CNS depressants.
Respiratory depression is a concern when too much is taken.
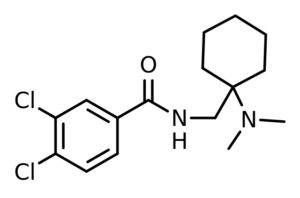
AH-7921 = Doxylam; 1-(3,4-dichlorobenzamidomethyl)cyclohexyldimethylamine
PubChem: 187760
Molecular formula: C16H22Cl2N2O
Molecular weight: 329.265 g/mol
IUPAC: 3,4-dichloro-N-[[1-(dimethylamino)cyclohexyl]methyl]benzamide
Contents
Dose
Oral (tentative)
Range: 10 – 150 mg (more often 40 – 100 mg)
Light: 5 – 20 mg
Common: 20 – 50 mg
Strong: 50+ mg
There aren’t enough reports to be confident in the doses. Even though some of the more reliably successful reports involve around 100 mg, there are physical safety concerns.
Routes
The drug is often taken orally, though there are reports of intranasal, sublingual, IV, and rectal administration.
Timeline
Oral (tentative)
Total: 6 – 8 hours (some reports indicate around 4 – 5 hours)
Onset: 00:15 – 00:45
Inhalation (tentative)
Total: 3 – 4 hours
Onset: 00:02 – 00:10
Experience Reports
Effects
Positive
- Relaxation
- Euphoria
- Mood lift
- Pain relief
Negative
- Respiratory depression
- Nausea
- Tachycardia or bradycardia
- Itchiness
- Drowsiness
- Constipation
Most reports seem to conclude with stating it’s not a particularly potent or interesting drug. It’s often not very euphoric and nausea is pretty common (to varying degrees).
There are some positive reports where it’s been used for analgesia or withdrawal relief. Sedation appears to be less prominent than with other opioids at common doses.
Animal Tests
AH-7921 was shown to provide analgesia with a potency similar to morphine.
For respiratory depression, it has been listed as both more and less potent than morphine. One report claimed it was 1.6x more potent for respiratory depression, meaning the negative effects may become an issue pretty close to the effective analgesic dose.
Chemistry & Pharmacology
Chemistry
It’s an N-substituted cyclohexylmethylbenzamide. The “AH” refers to Allen & Hanburys, which originally patented the drug.
AH-7921 is a derivative of dimethylaminocyclohexane, with the addition of a 3,4-dichlorobenzamide moiety.
Pharmacology
The primary activity seems to come from agonism at the u-opioid receptor (Ki: 10 nM), but involvement from the k-opioid system (Ki: 50 nM) does exist. Ki values were obtained from tests in guinea pig brain tissue.
It’s been found to alleviate naloxone and nalorphine-induced “withdrawal” syndrome. Naloxone also appears to block AH-7921’s effects in animals.
Most tests in animals found it was more potent than codeine and similar in potency to morphine. The minimum oral dose for suppressing pain response in canines was 1.25 mg/kg.
Metabolites
AH-7921 is primarily metabolized to desmethyl-AH-7921, which can then result in di-desmethyl-AH-7921.
History
Mid-1970s
The substance was designed and synthesized by researchers at Allen & Hanburys and the University of Aston. It was created as a potential analgesic and it was found to be the most active drug in its series.
Based on tests in animals, it was listed as having an analgesic ED50 of 0.55 mg/kg. The phenylquinone writhing test in mice gave it an ED50 of 0.45 mg/kg.
Allen & Hanburys completed a toxicity study that showed the LD50 in mice was 10 mg/kg (IV).
A report of its activity was first provided in the 1973 paper, “Anti-nociceptive effects of N-substituted cyclohexylmethylbenzamides.”
1976
The drug was patented by Allen & Hanburys, though it wasn’t long before research ended. A specific reason for the conclusion of research wasn’t provided, though some reports have suggested it was due to the drug’s addiction potential.
1980s
It was investigated a little further by a separate team. They found it provided its activity through the u-opioid receptor. The drug was again shown to be more potent than codeine, while being nearly equipotent to morphine.
1989 – 2010s
No studies were published.
2011
Discussion about the drug began online, with some posts suggesting it had been used to a limited degree prior to its appearance on the market.
2012
Authorities first reported its availability on the recreational market. This was followed by quite a few experience reports, non-fatal cases, and fatalities.
2012 – 2014
The majority of its use in the research chemical (RC) market occurred during this time.
In June 2012, it was first detected in the UK in 250 mg of white powder that had been purchased online. By the following month, Norway and Sweden brought the drug to the attention of the EU’s early warning system.
Between December 2012 and March 2013, Swedish authorities reported five non-fatal intoxications. From January 2013 to September 2013, those cases were followed by 10 deaths in Sweden involving AH-7921. Other drugs were detected in the fatalities, though AH-7921 was listed as the cause in two cases.
Japanese authorities reported its presence in some synthetic cannabinoid and cathinone products in 2013.
2015
By this point, the drug’s importance in the RC market appeared to have declined. Demand was down, fewer active sellers were reported, and there were fewer deaths.
2016
It became Schedule 1 in the US.
Legal Status
United States
Schedule 1
Also controlled (list may not be complete)
Brazil, China, Czech Republic, Finland, Israel, Netherlands, Norway, Poland, Romania, Sweden, UK
Safety
Not enough information exists to say what a reliably low-risk dose will be. Whatever safety issues do exist may become most prominent upon reaching the hundreds of milligrams.
Overdose
Respiratory depression and consciousness impairment will occur in an overdose. Based on the animal research, naloxone should have an effect.
Risky Combinations
Using any other CNS depressants (e.g. benzodiazepines, opioids, alcohol) will make it easier to develop respiratory depression, among other things.
Deaths
There have been a number of deaths connected to the drug, most of which involve other drugs that could be contributing factors. However, there are cases where the other drugs are only found at nontoxic concentrations. This may suggest AH-7921 is a major factor in some of the deaths.
Here are some of the case reports:
Group of Swedish cases
10 deaths reported. The drug was detected in femoral blood in nine cases.
Concentrations: 0.03 – 0.99 mg/L
None of the cases exclusively involved AH-7921. Among the other drugs detected were opioids, benzodiazepines, and antidepressants.
Nearly all of those affected were males in their 20s.
Group of UK cases
All of these occurred between January and November 2013. In none of the fatalities was AH-7921 the only drug detected.
Femoral blood range: 0.05 – 4.46 mg/L
Case 1
A 19-year-old male was found dead in bed by a friend. He had taken an unknown drug on the night of his death and on the preceding nights.
DXM, MPHP, and AH-7921 were detected. AH-7921 was presumed to be a major factor and the others weren’t detected in his blood.
Heart blood: 3.9 mg/L
Peripheral blood: 9.1 mg/L
Case 2
27-year-old.
Femoral blood: 0.821 mg/kg
Case 3 – Norway – December 2012
Polydrug use was involved.
Peripheral blood: 0.43 mg/L
Case 4 – Norway – August 2013
Polydrug was involved. It appears the substance was administered intravenously.
Case 5 – US
Other drugs appeared to be involved.
Peripheral blood: 9.1 mg/L
Case 6
Man in his early 20s. Codeine, 2-FMA, and 3-MMC were also detected.
Peripheral blood: 0.43 mg/L
Case 7
A young female was found dead at home by her boyfriend. Small bags labeled “AH-7921” and “Etizolam” were found at the scene.
Etizolam, phenazepam, diazepam, and oxazepam were all also detected.
Peripheral blood: 0.33 mg/L
References
(2015) AH-7921: the list of new psychoactive opioids is expanded
(2015) AH-7921: A new synthetic opioid of abuse
(2014) A Fatality Involving AH-7921
(2014) Lethal poisonings with AH-7921 in combination with other substances.
(2014) Fatal Intoxications Associated with the Designer Opioid AH-7921
(1983) Determination of receptors that mediate opiate side effects in the mouse.