4-Fluoroamphetamine is a substituted amphetamine that offers effects somewhere between those of amphetamine and MDMA. It’s been a member of the research chemical market since the late 2000s.
It has at times been a more popular research chemical because it does have a unique and potentially desirable effect profile.
Not enough is known about the drug to be sure about its safety level, particularly with chronic use.
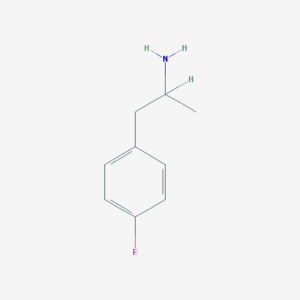
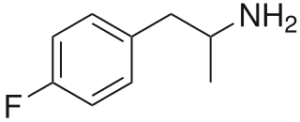
4-Fluoroamphetamine = 4-FA; 4-FMP; PFA; Para-Fluoroamphetamine; Molly’s Mosquito
PubChem: 9986
Molecular formula: C9H12FN
Molecular weight: 153.2 g/mol
IUPAC: 1-(4-fluorophenyl)propan-2-amine
Contents
Dose
Oral
Light: 25 – 50 mg
Common: 50 – 100 mg or 100 – 150 mg
Strong: 150+ mg
The common dose range depends on what effects you’re looking for. A lower amount (50 – 100 mg) is better for productivity, while a higher amount (100 – 150 mg) usually makes the drug more recreational.
Timeline
Oral
Total: 4 – 7 hours (at least somewhat longer for the stimulation vs. entactogen activity)
Onset: 20 – 40 minutes
Experience Reports
Effects
Positive
- Euphoria
- Mood improvement
- Music enhancement
- Increased talking
- Physical euphoria
- Disinhibition
- Increased empathy
- Increased sociability
Negative
- Bruxism
- Insomnia
- Appetite suppression
- Headache
- Anxiety
- Irritability
- Vasoconstriction
- Increased heart rate
- Increased blood pressure
It’s generally described as a cross between amphetamine and MDMA. The effects are cleaner and more entactogenic than amphetamine, but definitely not as entactogenic as MDMA.
The wakefulness is equivalent to or greater than MDMA, as is the case with some of the pro-social effects. Users don’t report the stimulation to be very forced or uncomfortable in most cases.
As was already mentioned, the effects are dose-dependent. It’ll be primarily like amphetamine under 100 mg and a bit more MDMA-like over 100 mg.
Users tend to report a calm mindset with the drug and the chance of anxiety or agitation appears lower than with amphetamine. It’s often very pro-social with common or strong doses. An MDMA-like floatiness may be present.
The effects often have a wave-like nature, meaning there can be short periods where it feels like you’re much closer to sober. 4-FA has a smooth onset period and typically doesn’t produce a negative comedown or strong negative day-after effects.
Duration
If you’re taking a more recreational amount, the main effects will last 4-5 hours and be followed by more amphetamine-like stimulation for a few more hours.
Productivity
It’s not going to be particularly useful as a productive stimulant when over 100 mg is used. Around 50 to 75 mg is reportedly useful as a productivity aid, similarly to common amounts of amphetamine. Also, it may be productive once the core entactogenic-like effects have worn off with higher doses.
Survey
A 2015 survey of 249 people in the Netherlands gives some idea of the typical effects and how 4-FA is used. Most people reported taking it in festivals, dance parties, and clubs. They primarily obtained it through friends or online shops.
77.1% used the drug because of its specific effects, not because of its legal status. Most participants first reported taking it around 2013.
Oral was the most common route of administration (92.8%). 100 – 150 mg was the most common dose range, though close to 20% used over 150 mg.
Participants said it lasted either 4 to 6 hours or 6 to 8 hours.
The actual effect scores were between those for MDMA and amphetamine.
Compared to amphetamine
- Less irritability
- Greater talkative and pro-social effects
- Lower “craving” score
- Similar for alertness and stimulation
- Greater euphoria and “connectedness to others”
- Greater “changes in sensory perception.”
Compared to MDMA
- Lower intensity, confusion, and dizziness
- Lower “craving”
- Lower euphoria and “connectedness to others”
- Lower “changes in sensory perception.”
Chemistry & Pharmacology
Chemistry
4-FA is a substituted amphetamine. It has a fluorine added to the core amphetamine structure.
The synthesis reportedly involves 4-fluoro-P2P (aka 4-fluoro-BMK).
Pharmacology
The drug functions as a monoamine releaser and reuptake inhibitor. Serotonin, dopamine, and norepinephrine are all elevated.
Its pharmacology (like the effects) is between that of MDMA and amphetamine. 4-FA has lower dopamine:serotonin and DAT:SERT ratios than amphetamine, but MDMA still has a greater effect on serotonin.
- DAT (IC50)
- 4 uM
- SERT (IC50)
- 20 uM
- NET (IC50)
- 0.4 uM
- Dopamine release (ED50)
- 200 nM
- Serotonin release (ED50)
- 1000+ nM
- Norepinephrine release (ED50)
- 50 nM
4-FA is 2x more potent than 4-chloroamphetamine as a dopamine reuptake inhibitor and 13x less potent as a serotonin reuptake inhibitor.
There appears to be some minor MAOI activity, though the potency is lower than 4-chloroamphetamine and 4-bromoamphetamine.
Corticosterone release is stimulated. 4-chloroamphetamine is also more potent in this regard. The release might not be connected to 4-FA’s serotonergic activity.
History
Early 1940s
It was synthesized. There’s no record of the drug being tested for years.
1960s
4-FA was studied in animals along with other para-substituted amphetamines.
2003
The first detection in Europe appears to have been reported in Germany.
2007 – 2008
This is when the drug’s current history truly begins. It began showing up throughout Europe, particularly in the Netherlands.
Initially it was detected as a result of sales originating with organized crime groups involved with amphetamine. They were using 4-fluoro-P2P to create an alternative drug.
2007 – 2013
While the substance was initially misrepresented as amphetamine and MDMA, that changed over time. The Netherlands reported an increase in intentionally purchased 4-FA after 2009.
More seizures in the rest of Europe were reported beginning around 2009. There were multiple cases of it appearing in “ecstasy” tablets in Switzerland during that year. It was considered a fairly popular NPS in Switzerland relative to other drugs in that market.
At least 12 European countries detected the drug by 2010 through forensic cases and consumer drug testing.
By 2013, it was more common for the drug to be intentionally purchased than misrepresented in the Netherlands. It was among the top four detected NPS in samples submitted to Dutch drug testing facilities. The others were 2C-B, methoxetamine, and 5-APB/6-APB.
A study of 3,335 last-month partygoers in the Netherlands revealed its use rate was nearly the same as ketamine and GHB.
Data from the Netherlands was similar to reports out of Spain, which also said 4-FA and 2C-B were among the top NPS.
2009 – 2011
15 forensic cases were reported in eastern Denmark. Most consisted of drugged driving, though there was one rape and one death of a known drug addict. The death was from polydrug use.
The rape involved a young woman who reported drinking ethanol and using cocaine. No cocaine was detected, only amphetamine and 4-FA.
2013 – 2015
Usage stats between countries have varied. They presumably trended down overall during this period. In Italy, it wasn’t a commonly seized NPS between 2013 and 2015. The seizure rate was similar to 2-FMA and 5-MeO-MiPT. Other NPS like 3-MMC, MDPV, and 4-MEC were higher on the list.
2016
16% of acute toxic effects reported to first aid stations at large-scale events in the Netherlands were attributed to 4-FA. This stat increased significantly from prior years.
- 2009 to 2011 – No toxic effects reported.
- 2012 – Under 1%
- 2013 – Under 1%
- 2014 – 2%
- 2015 – 11%
Legal Status
United States (as of June 2017)
Federally unscheduled.
State schedules: Arizona, Florida, Louisiana, and Virginia.
Controlled (list may not be complete)
Brazil, Canada (due to amphetamine analog status), China, France, Germany, Hungary, Israel, Italy, the Netherlands, Poland, Slovakia, Serbia, and the UK.
Safety
We don’t have enough information about 4-FA to tell how safe it’ll be in the long-term, especially with heavy chronic use. For this reason, it should be used infrequently at common doses and without combinations.
Lethal dose
This isn’t clear for humans, but we do have some animal data.
- Mouse
- 46 mg/kg IP (potential human equivalent of 3.7 mg/kg)
- Mouse (male)
- 150 mg/kg oral (potential human equivalent of 12.2 mg/kg)
- Mouse (female)
- 25 mg/kg oral (potential human equivalent of 2.0 mg/kg)
Evidence suggests 2.0 mg/kg and 3.7 mg/kg aren’t accurate. It could potentially be somewhere between 3.7 and 12.2 mg/kg.
Neurotoxicity
Animal studies suggest it has a relatively low potential for neurotoxicity. Even when serotonin depletion is seen, it’s short-lasting and isn’t indicative of damage.
One report in rats showed no signs of neurotoxicity 16 hours after an injection. Another report showed no decline in serotonin or 5-HIAA concentrations at 10 ug/kg. There was a temporary decline at 100 ug/kg.
The research suggests neurotoxicity isn’t as much of a concern as it would be for the bromo and chloro substitutions.
Frequency
It’s wise to avoid using the drug daily or near daily when taking light or low-common doses. When taking over 100 mg, it’s best to at least wait a month between uses, preferably a few months out of an abundance of caution.
Non-overdose cases
Case 1
- DUI leading to blood and urine sample
- The driver had dilated pupils
- Serum: 0.35 ug/ml
Case 2
- DUI
- Driver was believed to be under the influence of a psychostimulant given his restlessness and tremor.
- He claimed to have taken “speed.”
- Serum: 0.475 ug/ml
Overdoses
Case 1 – 2014
- 18-year-old male
- 5 hours after taking the drug he reported nausea, vomiting, shortness of breath, and chest tightness.
- He had used a “new street drug.”
- Two days prior he had received naltrexone and he was currently on fluoxetine and trazodone.
- Presented with an initial HR of 103 and temperature of 36.5°C (97.7°F)
- The symptoms worsened. He became very diaphoretic and hypoxic.
- Intubation and sedation followed.
- Testing revealed pulmonary edema and left ventricular hypokinesia.
- After he became hypotensive, dobutamine and epinephrine were administered.
- A 38.5°C (101.3°F) temperature occurred.
- This followed the administration of dopamine, dobutamine, milrinone, and furosemide.
- Cardiac symptoms improved by Day 3, but he wasn’t released for two weeks.
- He was given lisinopril and spironolactone daily after the incident.
- This group of symptoms was suggestive of acute cardiomyopathy triggered by catecholamine mechanisms or small vessel myocardial ischemia.
- A proposed diagnosis of reverse takotsubo syndrome was given.
- Question
- Did the drugs administered during his stay contribute to the problems given they could also lead to takotsubo syndrome?
- Concentrations (hours after administration)
- Urine: 64 ug/mL
- Serum: 0.118 ug/mL
Case 2 – 2015 (Laskowski, 2015 and Poklis, 2016)
- 27-year-old male
- Found agitated and lying on the street
- Alleged to have used 200 mg of 4-FA powder
- He presented to the ED four hours later
- PCP was present, but at a low concentration.
- Agitation, tachycardia, and hyperreflexia were noted upon presentation.
- He had hyperpyrexia of 41.4°C (106.5°F).
- This was addressed with ice water submersion and ICU admission.
- Given 28 mg of midazolam in total
- Ice water submersion
- Actively cooled for 22 min
- No shivering present
- Brought him to 99.3°F (37.4°C)
- The patient was allowed to leave a day later.
- Concentration
- Urine (4 hours post-admission)
- 285 ug/mL
- Urine (23 hours post-admission)
- 124 ug/mL
- Serum (23 hours post-admission)
- 1.4 ug/mL
- Urine (4 hours post-admission)
Case 3 – 2016
- 18-year-old female
- Presented to the ED after using:
- Two caps of Molly’s Mosquito (she believed it was “synthetic ecstasy)
- 110 mg of methylphenidate intranasal
- 800 mg of modafinil oral
- She initially felt euphoric, but then she developed headache, vomiting, nausea, lightheadedness, and diaphoresis.
- Only anxiety was reported by the time she reached the ED.
- Initial HR of 110 – 124 and 36.5°C (97.7°F) temperature
- Anxiety and tachycardia persisted during her time in the hospital.
- Treated with benzodiazepines, which was effective.
- Cardiomyopathy consistent with amphetamine/stimulant overdose appeared to be present.
- 36 hours post-administration
- Low ejection fraction of 10-15%, mild left ventricular dilation, and hypokinesis.
- A small troponin increase indicative of demand ischemia was present.
- 36 hours post-administration
- Concentration
- Urine: 285 ug/mL
Case Series
(Lonkhuyzen, 2016)
- DPIC in the Netherlands
- January to September 2016
- 36 4-FA exposures recorded
- Pronounced cardiovascular toxicity present in 11/36 (2 of which were analytically confirmed)
- One patient took 2 capsules of 4-FA, though no confirmation
- Had inverted Takotsubo cardiomyopathy
- 4 cases with analytic confirmation of 4-FA in blood or urine
- 3/4 took one capsule and subsequently had severe headache and cerebral hemorrhage
- 1/4 died
- Another patient died due to extensive bowel ischemia following chronic 4-FA use
- 3/4 took one capsule and subsequently had severe headache and cerebral hemorrhage
Risky combinations
- Other stimulants
- MAOIs
- Tramadol
Test Videos
References
(2017) Acute toxic effects related to 4-fluoroamphetamine
(2016) 4-Fluoroamphetamine in Serum and Urine from an Intoxicated Patient with Life-Threatening Hyperpyrexia.
(2016) Cardiovascular toxicity, cerebral hemorrhage, and mortality after 4-fluoroamphetamine
(2015) 4-Fluoroamphetamine in the Netherlands: more than a one-night stand
(2015) Ice water submersion for rapid cooling in severe drug-induced hyperthermia.
(2015) 4-Fluoroamphetamine(4-FA) – Critical Review Report
(2015) Takotsubo syndrome due to 4-fluoroamphetamine.
(2014) New phenethylamines in Europe.
(2014) Cardiogenic shock after use of fluoroamphetamine confirmed with serum and urine levels.
(2012) Isomers of fluoroamphetamines detected in forensic cases in Denmark.
(2012) Prevalence of new psychoactive substances: A retrospective study in hair.
(2012) Detection of the synthetic drug 4-fluoroamphetamine (4-FA) in serum and urine.
(2007) The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain.
(1995) Psychostimulant-like effects of p-fluoroamphetamine in the rat.